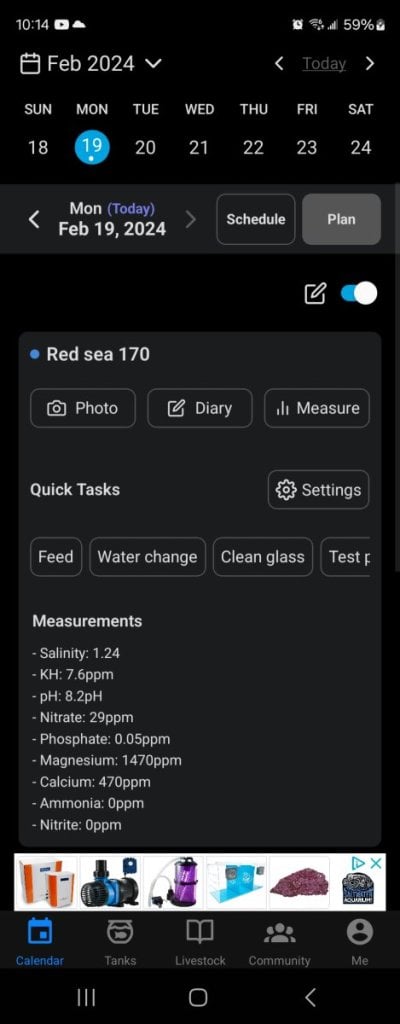
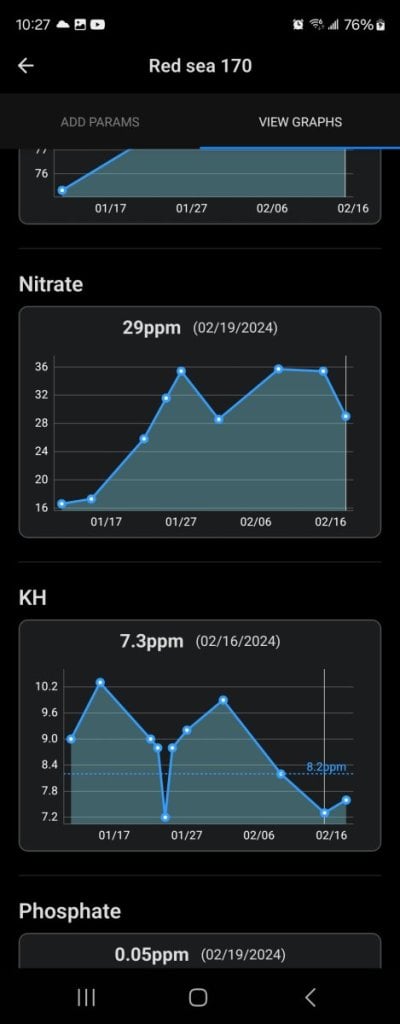
Ive been having problems keeping my kh stable i see it jump all over the place and i feel like it gets drained more then evrything else. Can anyone give me advice on what to do i am pretty new to reefing and dont want to wait until something goes wrong. In the pictures are my current parameters taken today a chart of my kh to see fluctuations and a look at my tank for reference. It is a red sea reefer 170 total water volume is 44 gallons including sump ask any questions and i will try my best to answer, i currently dose all for reef about 9ml per day split up into 2 times but like i said its harder to keep alk. Thank you evryone