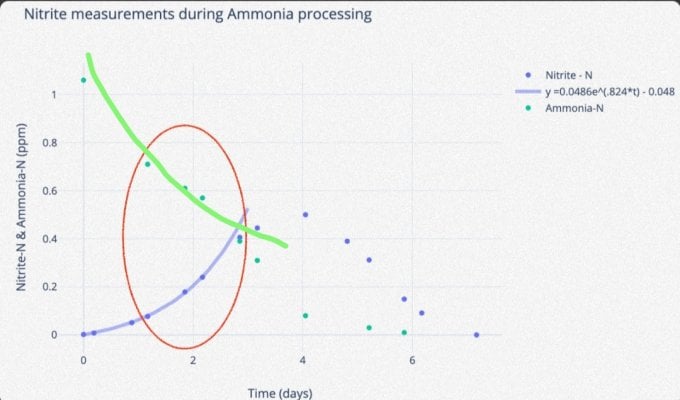
That's the tricky part, no? If the nitrospira does not die, but merely becomes dormant, I would expect the majority of them to become active at around the same time. If so, the graph should be rather linear after a initial lag time.Low death rate I guess.
In this case, we see a growth pattern. So perhaps death did occur.
Or I could be completely worng.