- Joined
- Dec 2, 2017
- Messages
- 165
- Reaction score
- 85
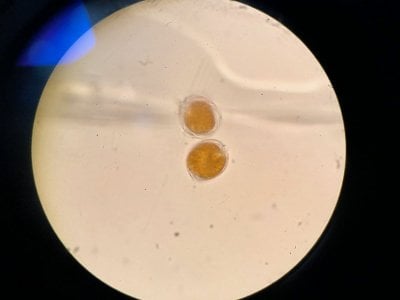
Did you run any of the tests from the first post to check it? That's the way to know. Even better is a microscope since they're so cheap...but you can start with the other diagnostics first to get the ball rolling.
This is the section you can use without a scope...
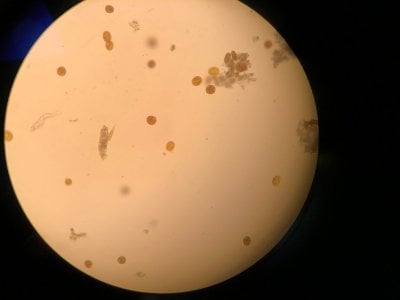
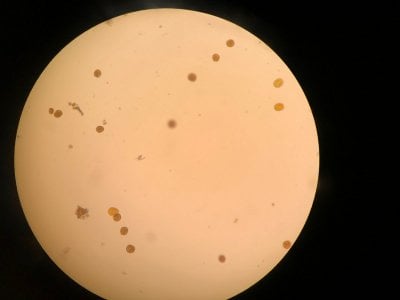
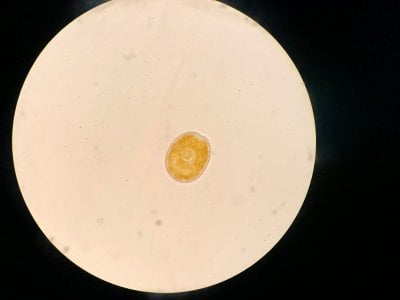
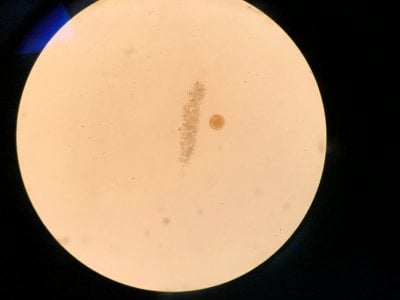
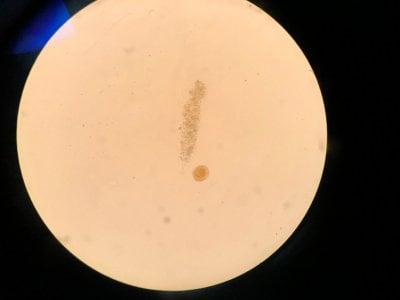
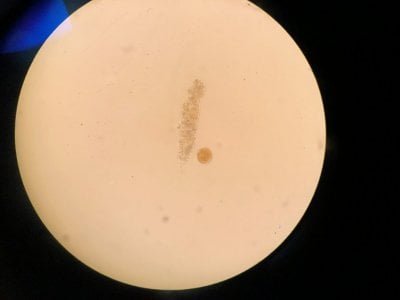
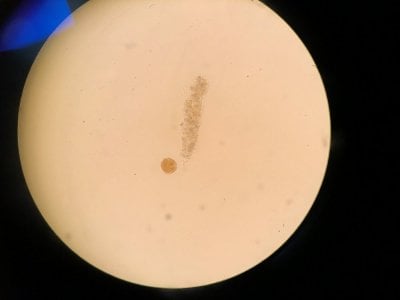
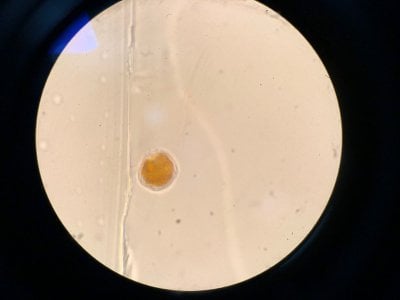
Yes the pictures I posted are from the microscope. I can't really find anything that looks similar to what I see. Doesn't look like dinos, cyano or diatoms to be honest.