Nonsense!I must be missing something here but I don’t see what was described in the video which was stable alk throughout the day. That claim was also made earlier in this thread and has not yet been demonstrated. What I see is a spike at the beginning of the day (when bolus is dosed) followed by decline in alk with the majority being used during the photo period.
That is what would be expected regardless of what chemical used for dosing (not considering ph). It is not, in my interpretation, what was claimed to happen in the video.
I for example dose sodium hydroxide at night. I test at the same time every morning (after final dose before lights on). I get a stable number each day I test. I don’t have an alk monitor, but I have zero doubt my graph would look very similar to yours up until my first dose at 1am. That graph would then show a gradual increase up until my final dose at 6am.
Fantastic claims require fantastic results. I don’t see anything fantastic here.
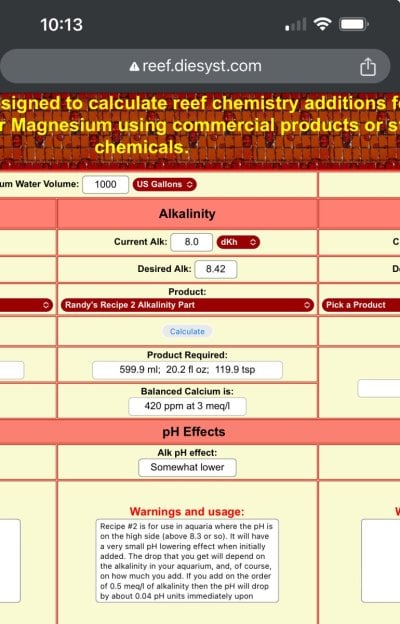
Have you seen the alk units?
clearly it’s gone right over your head!I must be missing something here but I don’t see what was described in the video which was stable alk throughout the day. That claim was also made earlier in this thread and has not yet been demonstrated. What I see is a spike at the beginning of the day (when bolus is dosed) followed by decline in alk with the majority being used during the photo period.
That is what would be expected regardless of what chemical used for dosing (not considering ph). It is not, in my interpretation, what was claimed to happen in the video.
I for example dose sodium hydroxide at night. I test at the same time every morning (after final dose before lights on). I get a stable number each day I test. I don’t have an alk monitor, but I have zero doubt my graph would look very similar to yours up until my first dose at 1am. That graph would then show a gradual increase up until my final dose at 6am.
Fantastic claims require fantastic results. I don’t see anything fantastic here.
There is a 0.2dkh swing over the whole day!!!
After dosing 600ml alk solution, where is the ALK spike you speak of??