- Joined
- Sep 21, 2018
- Messages
- 7,571
- Reaction score
- 7,962
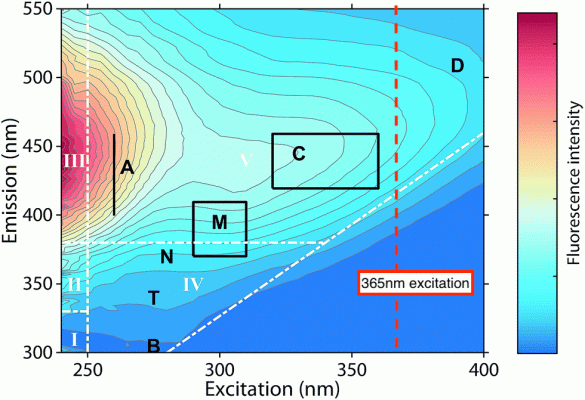
One point to consider is that the data was collected from a system containing ~10 oz of fish (!) in 15 gallons of water. Does similar chemistry happen in more dilute systems like a reef aquarium?Nice paper.
I don't know what this means in terms of what it says about the origin material or processes that create this DOC....
"Ultrafiltration of the culture water revealed that more than a half of
the total organic substances existed as organic carbon with a molecular
weight of the order of 10^4....
The fractionation of dissolved organic matter in
coastal seawater by ultrafiltration has shown that the fraction with a
molecular weight of an order of 10^4 usually accounts for the largest proportion of the total DOC (Ogura, 1974). "
What sorts of things are aquarium food inputs that are organics greater or equal to 10^4 molecular weight?
Or are breakdown processes making larger weight organic waste out of smaller stuff?