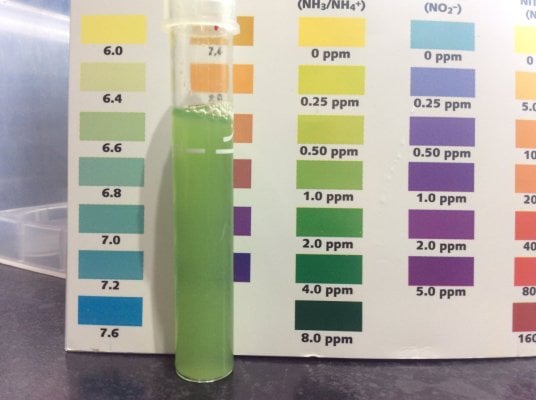
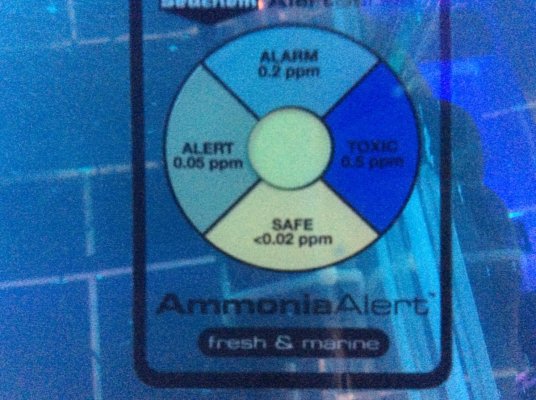
12 hrs after adding 2ppm ammonia, bloom still well underway.I´m awaiting your next test result. If you read my 15 steps - you will see that I suggest a PO4 dose too. (not vinegar though) Even the autotroph nitrificators need PO4 !
Generally adding of DOC will favour heterotrophs and disfavour the autotrophs. However the second stage bacteria - when they kick in - will convert produced NO2 quickly into NO3. I have seen 1- 2 mg/L NO3 been converted into NO3 in less than 10 hours when it kicks in
Sincerely Lasse
I might just ditch the water, see what the rock can do without all that 3 dimensional stuff going on it the water, where I’m sure is where most/all of the activity is taking place.