Thought I’d go “live bacteria” route for cycling a QT as the wife is itching to get a few fish in her tank. The most available bacteria by me was Fritzyme 9, so that’s what I went with.
Tank, ceramic rock, heater, Circulation pump were all bleached for 24 hrs then allowed to dry for 24 hrs prior to start up.
1) 40 liters of RODI / saltwater (Tropic Marin Classic) was added to the tank at 30ppt.
2) Tank heated to 26C
3) Added 50mls white vinegar (tank pH at 7.4 to 7.6ish), a bit vague but didn’t fancy messing with pH probes.
4) Added TriSodium Phosphate to 0.5ppm tested Hanna/API combo
5) Added Fritzyme 9 (8 ounce) as suggested by fritz for new startups.
6) Added 2ml Dr Tim’s ammonia.
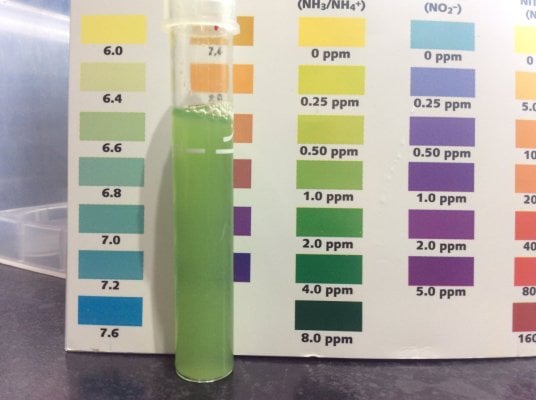
The TAN according to API was good enough on 2ppm (photo is next to a freshwater card so you can ignore the color chart)
Tank tested this morning, a little over 18 hrs after bacterial addition has showed a 50% reduction in NH3/NH4, an increase in Nitrite and pH. In particular pH is now above 7.8
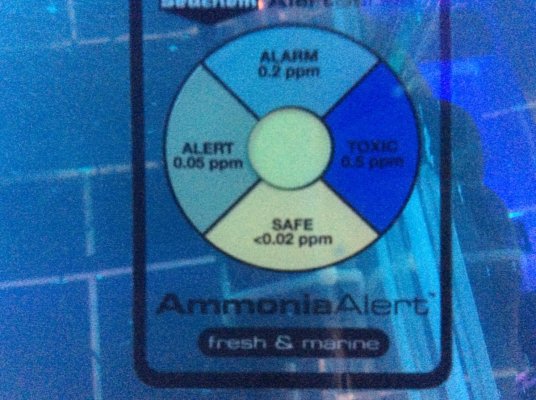
The Seachem ammonia alert has only now started to indicate a slight tinge, presumably due to the pH increasing, but still safe.
Tank is cloudy.
Fuzzy pics;



Tank, ceramic rock, heater, Circulation pump were all bleached for 24 hrs then allowed to dry for 24 hrs prior to start up.
1) 40 liters of RODI / saltwater (Tropic Marin Classic) was added to the tank at 30ppt.
2) Tank heated to 26C
3) Added 50mls white vinegar (tank pH at 7.4 to 7.6ish), a bit vague but didn’t fancy messing with pH probes.
4) Added TriSodium Phosphate to 0.5ppm tested Hanna/API combo
5) Added Fritzyme 9 (8 ounce) as suggested by fritz for new startups.
6) Added 2ml Dr Tim’s ammonia.
The TAN according to API was good enough on 2ppm (photo is next to a freshwater card so you can ignore the color chart)
Tank tested this morning, a little over 18 hrs after bacterial addition has showed a 50% reduction in NH3/NH4, an increase in Nitrite and pH. In particular pH is now above 7.8
The Seachem ammonia alert has only now started to indicate a slight tinge, presumably due to the pH increasing, but still safe.
Tank is cloudy.
Fuzzy pics;
































