Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
So is this a change of position from the 'every reefer knows that dosing bicarbonate does not increase pH' statement you'd made previously?
Are you being sarcastic?
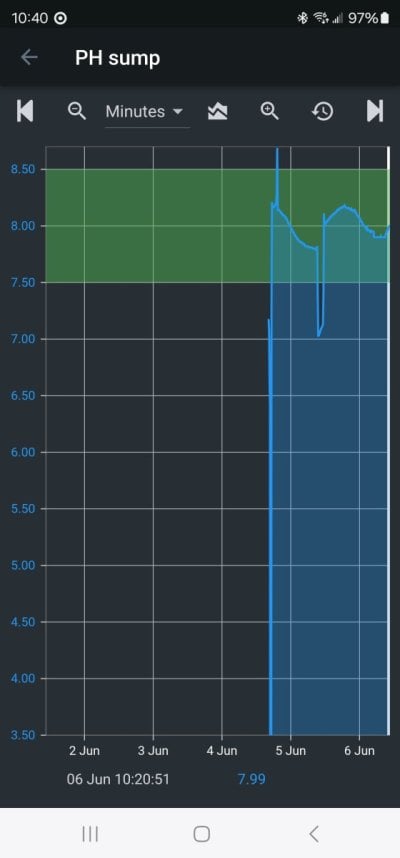
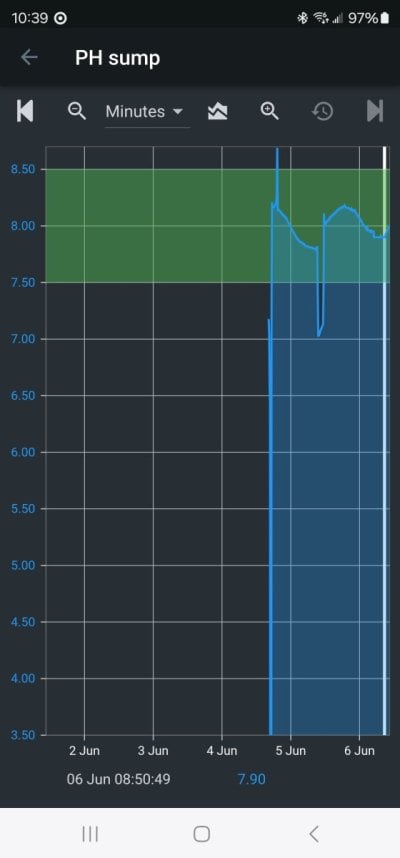
Every reefer knows (or can test) that when first added, bicarbonate lowers pH. I tested it and published it long ago.
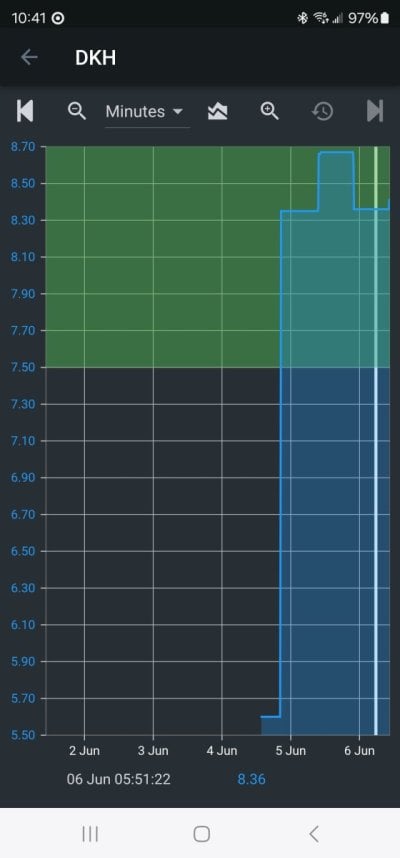
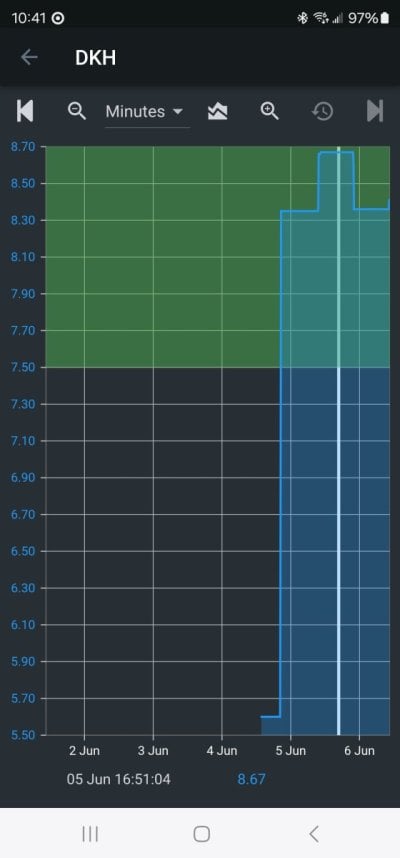
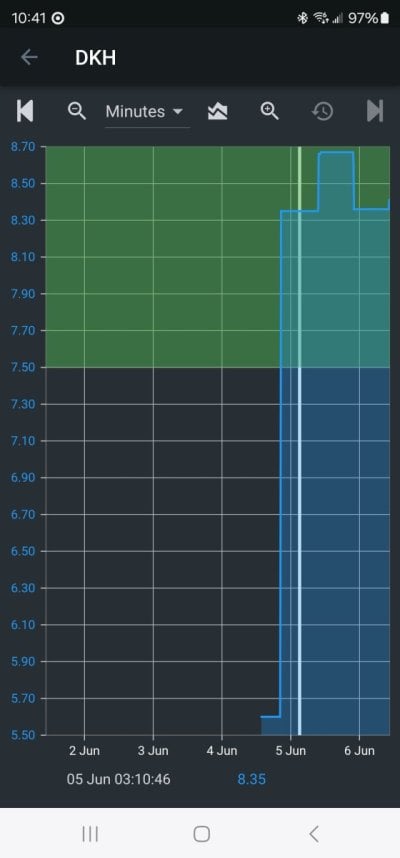
Every reefer knows or should know that seawater that is in equilibrium with air has a pH that only depends on the alk. Higher alk leads to higher pH. That is both well established chemical theory and I also measured it and published the data years ago.
Both of these effects were posted by me early in this thread, along with the exact data.
In summary:
1. When first added:
Bicarbonate lowers pH
Carbonate raises pH
Hydroxide raises pH about twice as much as carbonate
2. When that water begins to equilibrate with the air, the pH move in the direction of what it would have with full equilibration.
3. Tank water dies not stay in equilibrium with the air, and equilibrates fairly slowly, hence the reason that the nature of the alk additive can impact pH at all.