- Joined
- May 22, 2016
- Messages
- 6,970
- Reaction score
- 10,747
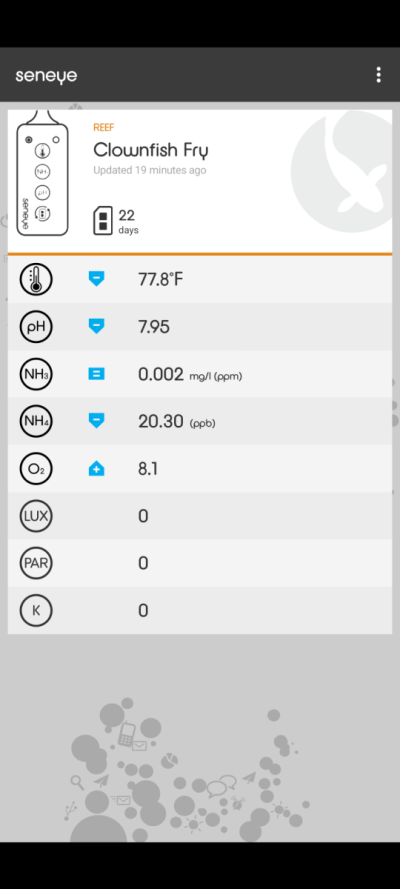
Always jealous of the trained chemistry nose.Here is my sniff report.
For both products, the aroma did seem that intense, snorting the head space was needed rather then wafting it towards my nose to get a good whiff.
Aqueon - dead snail aroma
Prime - rubber tire aroma with overtone of dead snail
Such a useful analytical tool.