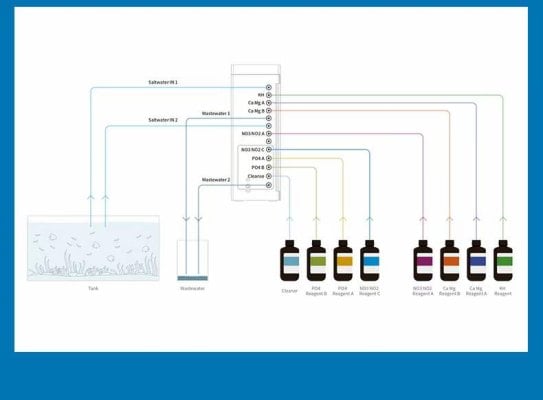
I forgot to add the detail that I am storing my dry indicators in brown glass and away from light as was recommended.What do you want to preserve them from?
If they are dry, air oxidation is the main issue. Sealing them well is the best way. Hach uses metal foil for this purpose. Moisture also facilitates the oxidation. In a few situations, changing physical form might be an issue, if a more soluble crystal form slowly converted to a less soluble one. Moisture sometimes facilitates that conversion. Pharmaceuticals sometimes include oxygen or moisture absorbing devices in prescription bottles for these reasons..
Some liquid reagents might be low enough in concentration and have other characteristics that may allow microbial growth. How to deal with that would be very case by case.
In a very few instances, light (typically low wavelength UV or blue) can cause breakdown of chemicals. Dyes might slowly break down when absorbing their specific wavelengths since that can put them into an excited electronic state that may be more prone to degradation. .
Steve