Just recapping the progress.
July 8th. 2024
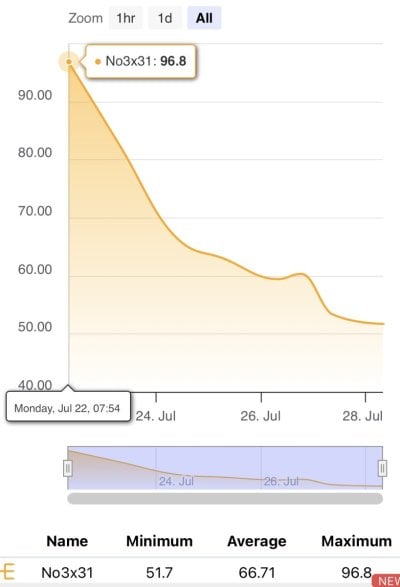
No3 Levels: 130ppm down to 60ppm
5.75l of media = 260ml/min. ORP: -200
Effluent: 0ppm @ 260ml/min
Too much media, removed some on July 26th when DT reached 60ppm No3.
July 26th. 2024
No3 Levels: 45ppm
2.6l of media = 175ml/min. ORP: 0
Effluent: 6.5ppm @ 170ml/min
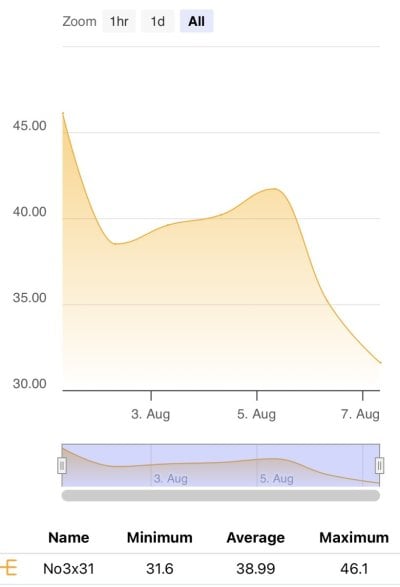
No3 stabilized and held at ~40ppm, starting to rise very slowly in DT, now 45ppm. Feeding heavy. Could be No3 bound in rock.
August 5th. 2024
No3 Levels: 40ppm*
4l of media = 200ml/min. ORP:-65
Effluent: ?ppm @ 200ml/min
Added another 1.4l of sulfur media. Haven’t tested effluent yet.
No3 Levels visualized by Trident NP on apex graphs:
July 8th. 2024
No3 Levels: 130ppm down to 60ppm
5.75l of media = 260ml/min. ORP: -200
Effluent: 0ppm @ 260ml/min
Too much media, removed some on July 26th when DT reached 60ppm No3.
July 26th. 2024
No3 Levels: 45ppm
2.6l of media = 175ml/min. ORP: 0
Effluent: 6.5ppm @ 170ml/min
No3 stabilized and held at ~40ppm, starting to rise very slowly in DT, now 45ppm. Feeding heavy. Could be No3 bound in rock.
August 5th. 2024
No3 Levels: 40ppm*
4l of media = 200ml/min. ORP:-65
Effluent: ?ppm @ 200ml/min
Added another 1.4l of sulfur media. Haven’t tested effluent yet.
No3 Levels visualized by Trident NP on apex graphs:


Last edited:






















