I mixed mine in an old 1g Mott's apple juice bottle and it melted the plastic and got NaOH all over my laminate floors. Guess I lost my security deposit.
Thats not good! Wonder if these jugs will do the same. It comes in tomorrow so we shall see.
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
I mixed mine in an old 1g Mott's apple juice bottle and it melted the plastic and got NaOH all over my laminate floors. Guess I lost my security deposit.

hi please can you confirm a cup is 128g? thanksIn a previous thread, I posted a true two part DIY recipe:
https://www.reef2reef.com/threads/new-diy-two-part-recipes-with-higher-ph-boost.344500/
But some folks may want to just swap the new ingredient into my 2/3 part recipe (as used by BRS, for example).
Here's the original recipe link (which has a lot more discussion on the details and rationale):
http://reefkeeping.com/issues/2006-02/rhf/index.php
The new recipe is shown below. It has about twice the pH boost of the original recipe (#1) and should be added to a very high flow area. Initial cloudiness (magnesium hydroxide) is expected, but it should disperse and dissolve. If not, stop using it and figure out why.
Alk part
Add 283 grams of sodium hydroxide to 1 gallon of fresh water. It will get quite warm. Make sure it doesn't soften your container. This solution will contain about 1,900 meq/L of alkalinity (5,300 dKH). BE CAREFUL WITH THIS SOLUTION: IT HAS A pH ABOVE 14. Do not get it in your eyes or on your skin.
Calcium part
Dissolve 500 grams (about 2 ½ cups) of calcium chloride dihydrate (such as Dowflake 77-80% calcium chloride or ESV calcium chloride; see below for substitutes and sources) in enough water to make 1 gallon of total volume. You can dissolve it in about ½ gallon of water, and then pour that into the 1 gallon container and fill it to the top with more freshwater. This solution has about 37,000 ppm calcium.
Magnesium part
Dissolve Epsom salts (magnesium sulfate heptahydrate (3 cups) and magnesium chloride hexahydrate (5 cups) in enough purified freshwater to make 1 gallon total volume. There will likely be a precipitate that forms even if you fully dissolve both ingredients separately. That precipitate is calcium sulfate (calcium as an impurity in the magnesium chloride and sulfate from the Epsom salts). It is fine and appropriate to dose the precipitate along with the remainder of the fluid by shaking it up before dosing.
This solution is added much less frequently or in lower volume than the other two parts. Add 16% as much as the other two parts. Over the time you add 1 gallon of the others, 1 add 610 mL (2 ½ cups) of this solution. You can add it all at once or, preferably, over time as you choose, depending on the aquarium's size and set up. Add it to a high flow area, preferably a sump. In a very small aquarium, or one without a sump, I suggest adding it slowly.
236g I believehi please can you confirm a cup is 128g? thanks
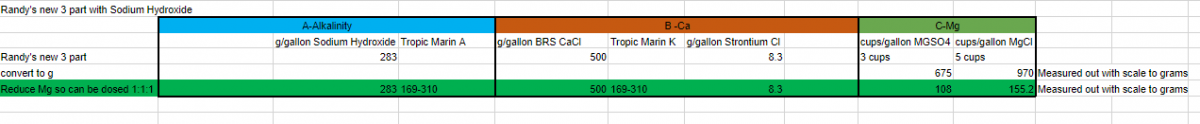

Hi yes thats what I thought but the mag recipe uses cups and I wondered why it’s not in g’s?

@Randy Holmes-Farley Is it best to run through our Soda ash Alk mixture in my dosing containers before switching to the new NaOH mixture or can we just start topping that solution off with the NaOH as I convert over? Wasn't sure if there was going to be a reaction issue between the soda ash and the NaOH. Thank you
Awesome. Thank you for the confirmation. Does my chart a couple posts up make sense for the dilution so you can dose 1:1:1? ThanksIt's OK to start topping off the containers, as long as you are making the same alk potency using the hydroxide. if you are making it more potent in alk per mL, then I'd wait to avoid possible precipitation of sodium carbonate.

Awesome. Thank you for the confirmation. Does my chart a couple posts up make sense for the dilution so you can dose 1:1:1? Thanks
def alk_dose(pH, dose_amount):
if pH >= 8.3:
dose(dose_amount, Na₂CO₃)
elif 8.2 < pH < 8.3:
dose(0.5*dose_amount, Na₂CO₃)
dose(0.5*dose_amount, NaOH)
else:
dose(dose_amount, NaOH) elif 8.2 < pH < 8.3:
pH_factor = (pH - 8.2)*10
dose(pH_factor*dose_amount, Na₂CO₃)
dose((1-pH_factor)*dose_amount, NaOH)
Couple questions...
How likely is it, while using NaOH, to reach a point where the pH boosting effect is too high to continue using it as the only source of Alkalinity?

