- Joined
- Jul 16, 2009
- Messages
- 5,071
- Reaction score
- 8,108
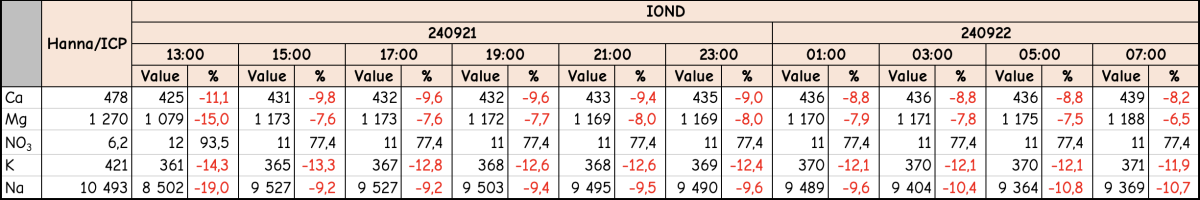
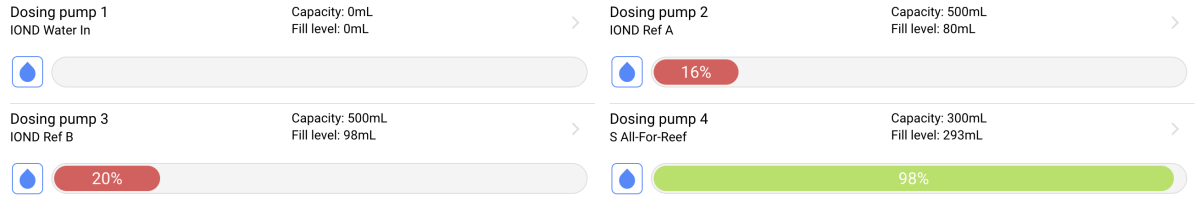
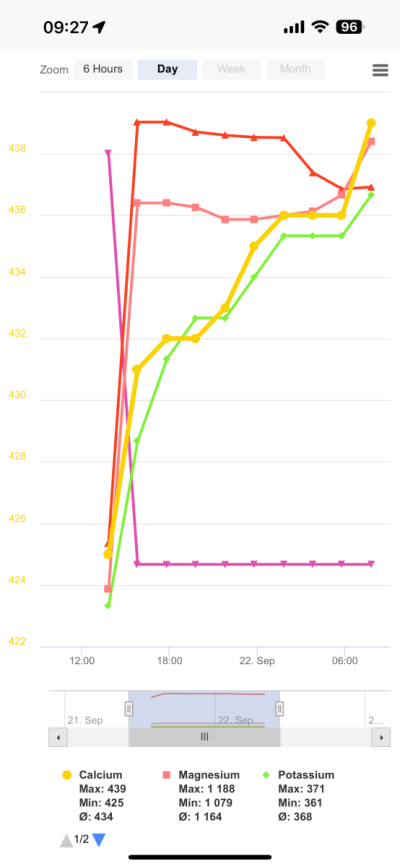
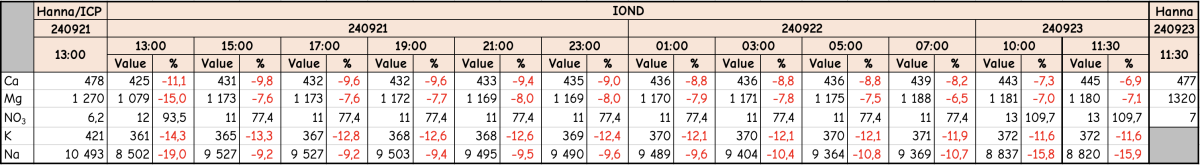
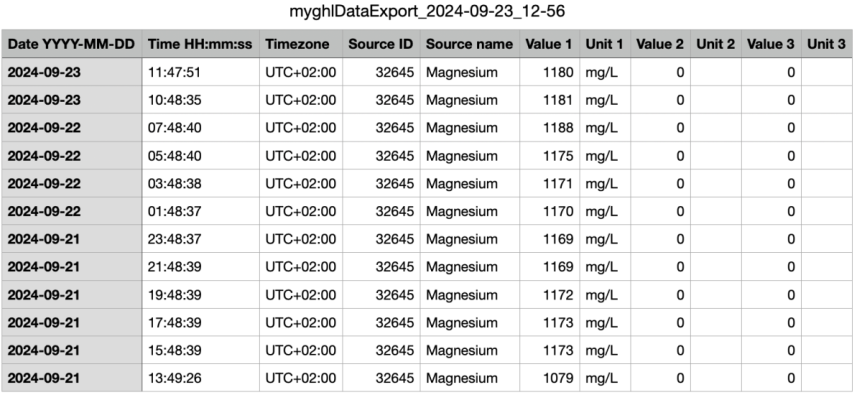
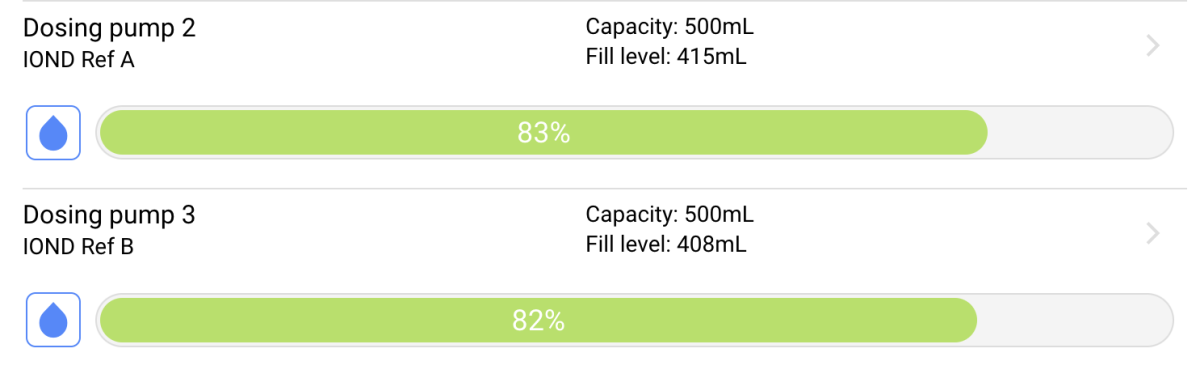
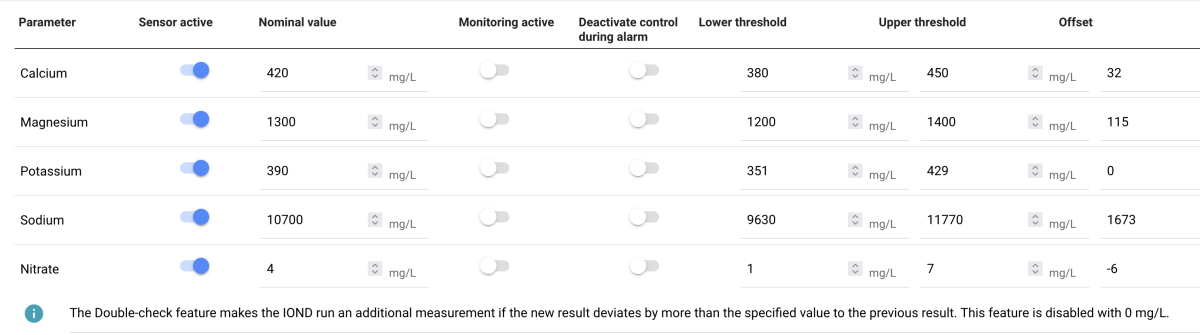
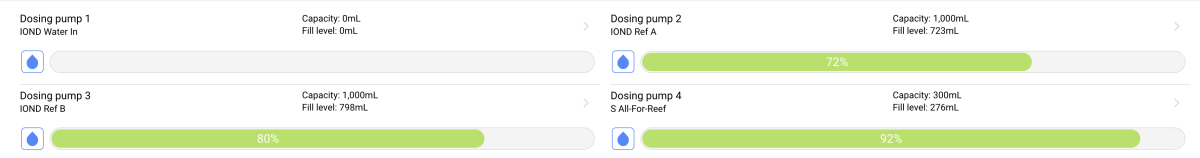
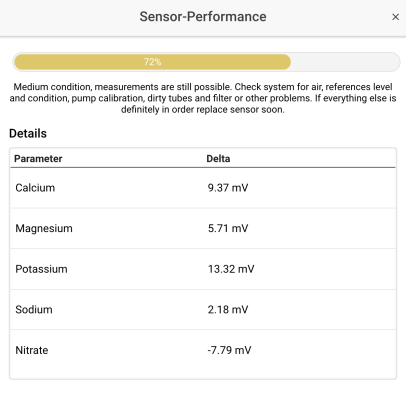
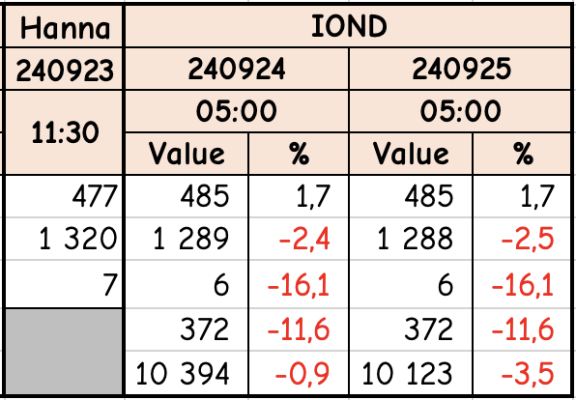
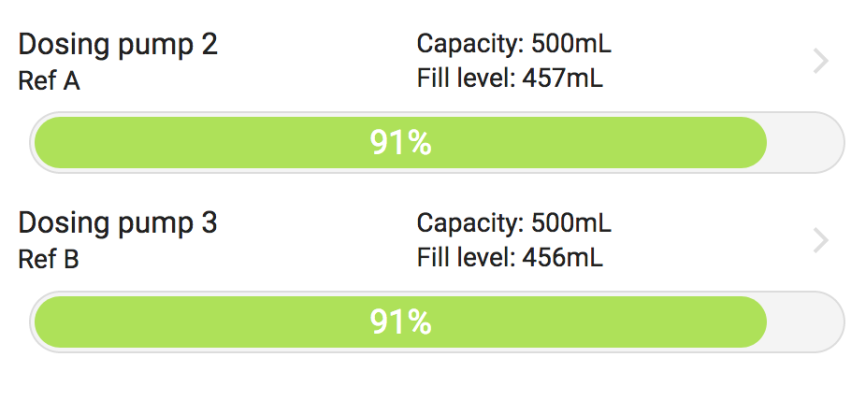
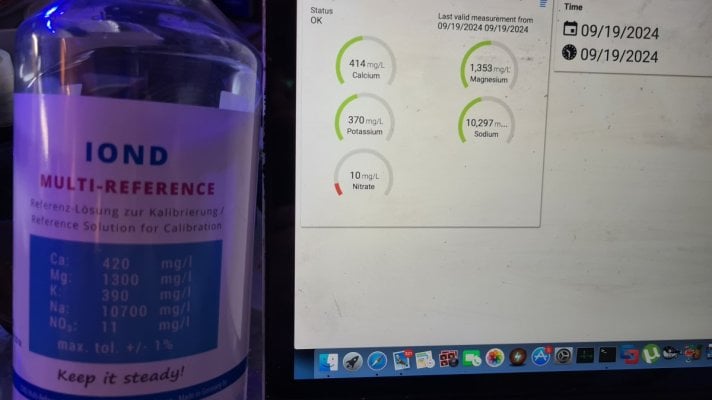
I was (honestly) shocked when I did my back of napkin math on the unit and consumables cost, best case with minimal failed tests, advertised probe life, conditioning, etc. If was already kind of a deal breaker for me, but consumption over the spec and possible shorter probe life... I decide to hard pass.
Looking forward to your continued test results though.
Looking forward to your continued test results though.