Rick Mathew
Valuable Member
View BadgesPartner Member 2024
Excellence Award
Article Contributor
Hospitality Award
Ocala Reef Club Member
Thanks!
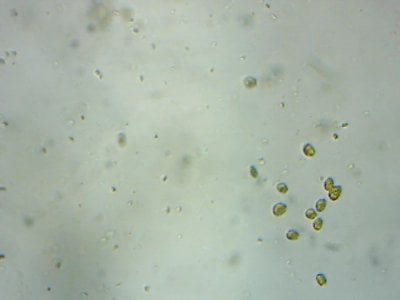
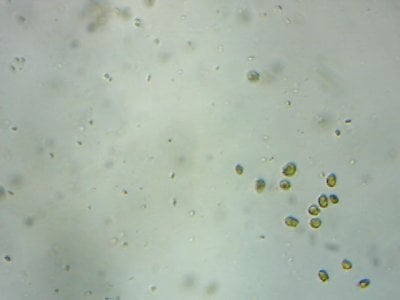
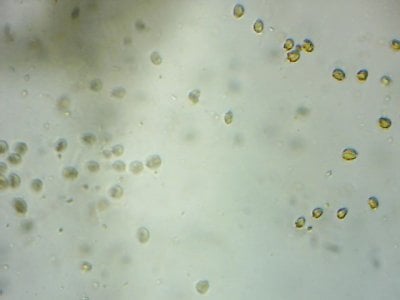
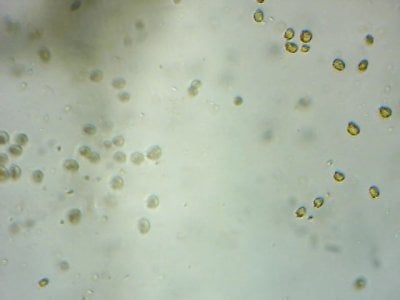
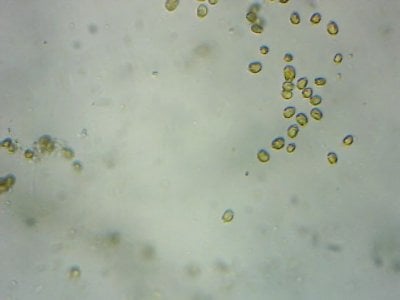
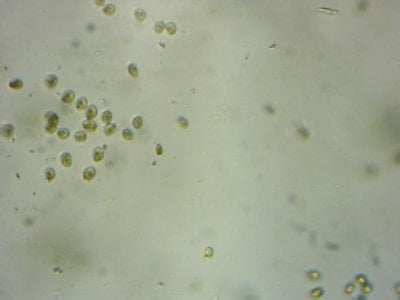
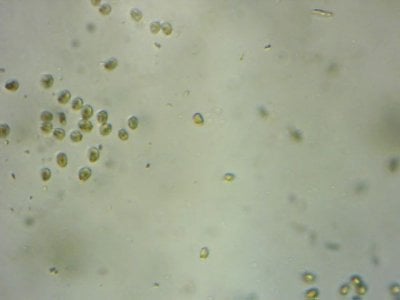
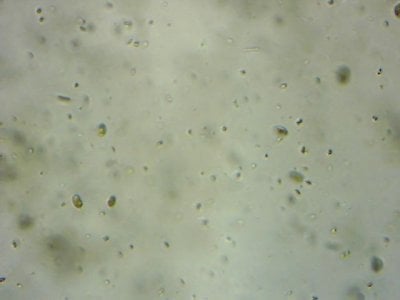
Yep. I feel like most tanks (in the dino threads at least) would be better off with more nutrients and better herbivores to manage algae, rather than trying to manage algae from the nutrient side.
Totally agree!