How do you prevent water in the skimmer should it fill up being drawn into the scrubber?
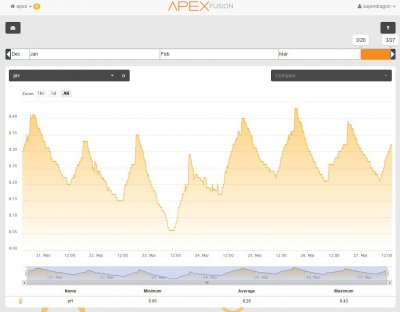
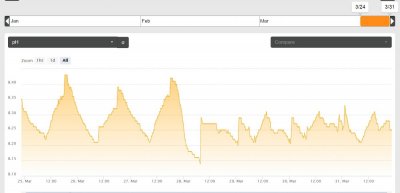
View attachment 501279
Skimmer cup, black silicone airline tubing x 2, then reactor.
We find that adding water to the reactor does not last that long. These are the instructions from brs, click on more and "directions"
http://www.bulkreefsupply.com/brs-universal-co2-scrubber.html
or
What I did above. Media lasts longer with above.