- Joined
- Mar 17, 2017
- Messages
- 845
- Reaction score
- 1,310
I just cleaned my kalk reactor, but the potency of my slurry is declining very rapidly - any idea what is causing this?
I have am avast marine k1 kalkwasser reactor. typically i fill that up with aobut 50 to 100 ml of kalk powder (ESV) - (sorry i measure my kalk by pouring it into an empty 50 ml conical tube and adding this tube to the reactor). This usually keeps the ph inside the reactor for 12-11.5 for a good week or so.
Yesterday i took the kalk reactor offline and cleaned it, and removed any of the powder in the bottom. I filled it out with cold RODI water (45f) put it back online in the sump (water temp is 78f) and adding 2 tubes of kalkwasser; this raised the ph quickly to 12, but then it declined to below 11 in the span of a couple of hours. i then added 2 more conicals of kalkwasser. PH went to 12 again for a couple of hours, but then decline quickly to below 10. I added two more conicals this morning and again it only momentarily went to 12 then decline again. Water in the kalk reactor looks normal (whitish), and i see kalk at the bottom of the reactor.
The reactor is fed with a continuous doser kamoer at about 4 ml/min with RODI water.
Not sure why the potency is declining so quickly... is it air in the reactor ? is it related to temp of RODI water initially used to fill reactor. Should i just keep adding kalk ?
thanks for your help... not really understanding what is causing this...

as an aside - i am not sure if this is realted but my tank's PH dipped as well after the clean up, but i am thinking this is a cuase of the unsaturated kalk in the reactor vs. something external affecting the PH in both the tank and kalk reactor...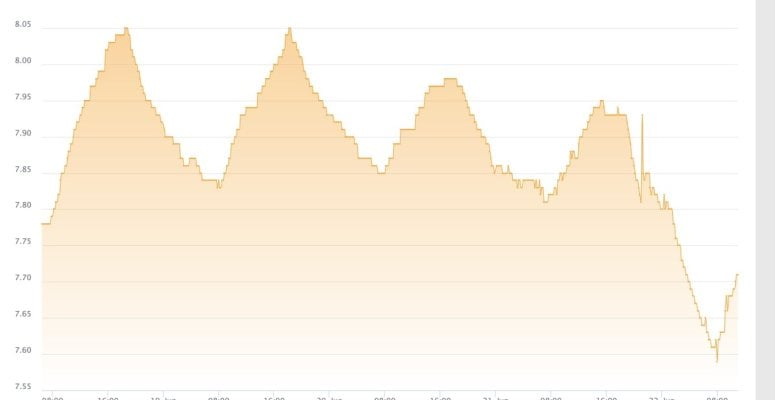
I have am avast marine k1 kalkwasser reactor. typically i fill that up with aobut 50 to 100 ml of kalk powder (ESV) - (sorry i measure my kalk by pouring it into an empty 50 ml conical tube and adding this tube to the reactor). This usually keeps the ph inside the reactor for 12-11.5 for a good week or so.
Yesterday i took the kalk reactor offline and cleaned it, and removed any of the powder in the bottom. I filled it out with cold RODI water (45f) put it back online in the sump (water temp is 78f) and adding 2 tubes of kalkwasser; this raised the ph quickly to 12, but then it declined to below 11 in the span of a couple of hours. i then added 2 more conicals of kalkwasser. PH went to 12 again for a couple of hours, but then decline quickly to below 10. I added two more conicals this morning and again it only momentarily went to 12 then decline again. Water in the kalk reactor looks normal (whitish), and i see kalk at the bottom of the reactor.
The reactor is fed with a continuous doser kamoer at about 4 ml/min with RODI water.
Not sure why the potency is declining so quickly... is it air in the reactor ? is it related to temp of RODI water initially used to fill reactor. Should i just keep adding kalk ?
thanks for your help... not really understanding what is causing this...
as an aside - i am not sure if this is realted but my tank's PH dipped as well after the clean up, but i am thinking this is a cuase of the unsaturated kalk in the reactor vs. something external affecting the PH in both the tank and kalk reactor...



















