- Joined
- May 17, 2020
- Messages
- 185
- Reaction score
- 245
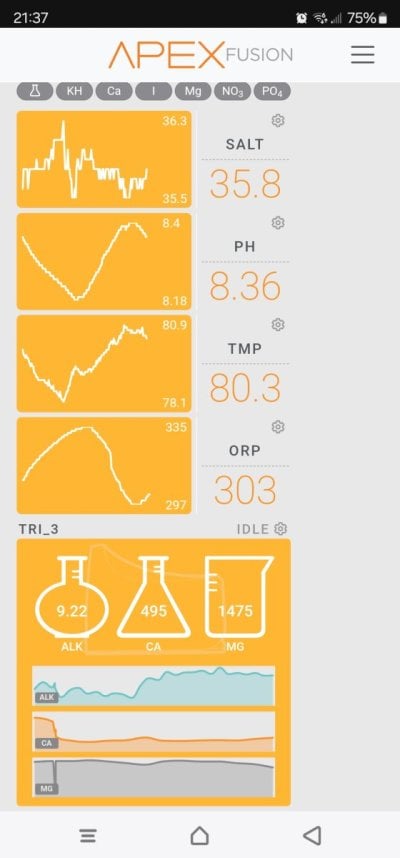
So, I've been using Kalkwasser for about a year half...and feel like I have a pretty good grip.
My issue is - my Calcium consumption.
I usually top up my kalk stirrer once a week, as I see the pH and Alkalinity start to drop, signifying the kalk is being used up. Issue is, my Calcium consumption has decreased a lot (due to coral lose) and adding more kalk, increases my Calcium level too - which, I don't want.
Someone mentioned to me - about Sodium Hydroxide. PH & Alk boost - without the Calcium. PERFECT!
Issue is..I dont really know where to start. I've read Randy's DIY - can get food grade SH and seem easy enough to mix with RO.
Do you dose it? I'm reading it warms up, when mixing - does it stay warm?
I mean - Kalkwasser I'm adding 24/7 - can I dose SH over a 24hr period too? Does the potency drop off, like Kalk or stay stable - to the end?
My issue is - my Calcium consumption.
I usually top up my kalk stirrer once a week, as I see the pH and Alkalinity start to drop, signifying the kalk is being used up. Issue is, my Calcium consumption has decreased a lot (due to coral lose) and adding more kalk, increases my Calcium level too - which, I don't want.
Someone mentioned to me - about Sodium Hydroxide. PH & Alk boost - without the Calcium. PERFECT!
Issue is..I dont really know where to start. I've read Randy's DIY - can get food grade SH and seem easy enough to mix with RO.
Do you dose it? I'm reading it warms up, when mixing - does it stay warm?
I mean - Kalkwasser I'm adding 24/7 - can I dose SH over a 24hr period too? Does the potency drop off, like Kalk or stay stable - to the end?





















