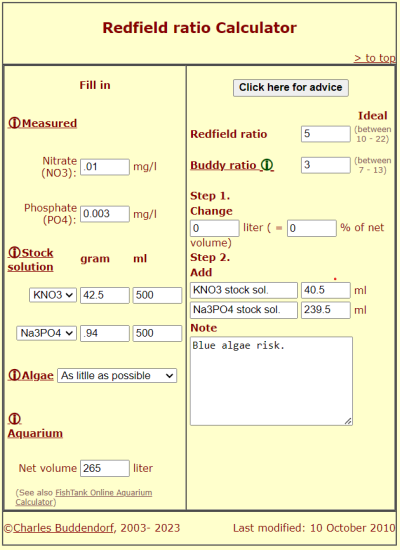
As my tank is growing I'm fighting with very low phosphate and nitrate. I have been feeding more and adding reef roids and AB+ but still have very low numbers. Just received my Na3PO4 and Ca(NO3)2 from Loudwolf. I have looked for a calculator/recipe but can't find one for these reagents. Any help would be appreciated. approx 85 gal water volume and almost undetectable values for both.
Thanks!!!
P.S. Also dose Kalk through ATO
Thanks!!!
P.S. Also dose Kalk through ATO
Last edited: