- Joined
- May 22, 2016
- Messages
- 6,970
- Reaction score
- 10,747
Density/specific gravity measurements of aquarium water can make people a little crazy because if you have two devices they are going to give you different results. So you get a third, or buy a calibration standard - and the "which method is most trustworthy?" game goes on. Part of the frustration is that it's literally the most fundamental parameter of our salt water - how much salt is in it?
I ran across a pycnometer for the first time a few months ago. And I eventually got one (two actually - 100mL and 10mL) to play with. In theory, a pycnometer and a good scale ought to give you a fundamental and highly trustworthy way to measure specific gravity.
It's not such an unusual an apparatus - it's just a special kind of volumetric flask made so that at a given temperature it can only possibly hold one volume of liquid. My 100mL pycnometer has a "certified volume" of 99.727mL at 20C.

So you can either work at that specific temp, and base your calculation off that certified volume, or - my preference is to just have tank water, distilled water and the pycnometer all at the same temp (water bath) and use specific gravity instead of density. For density I need exact volume - which means I need to know the temp and use the certified volume or measure it myself. For specific gravity, I just need a repeatable volume, distilled water and aquarium water at the same temp, and a scale. Since SG is fundamentally just a ratio, it cancels out a lot of measurement errors like if the flask volume is slightly off.
Here's how you fill it. You add liquid to the neck and then push the ground glass top stopper in place, it forces excess water up through a capillary and overflows, then you wipe off all excess. So the volume of liquid contained is pretty exact, and very repeatable.
Video of the filling process here
I wanted to see how good the precision was using this 100mL pycnometer, So I checked to see if it could measure the increase in salinity due to evaporation over 6 hours.
I took a few water samples over 6 hours, sealed them and put them in a tap water bath at room temp (24C) along with a sample of distilled water. after the last sample sat in the water bath for over an hour, I used the pycnometer to measure all the samples on a scale.
If the volume and temp are the same - specific gravity is simply the ratio (mass of sample / mass of pure water). So that's all I need.
I actually used two scales - the best one I have access to, goes to 0.0001g and a very not fancy centigram scale, goes to 0.01g.
Here's the measured masses in grams.
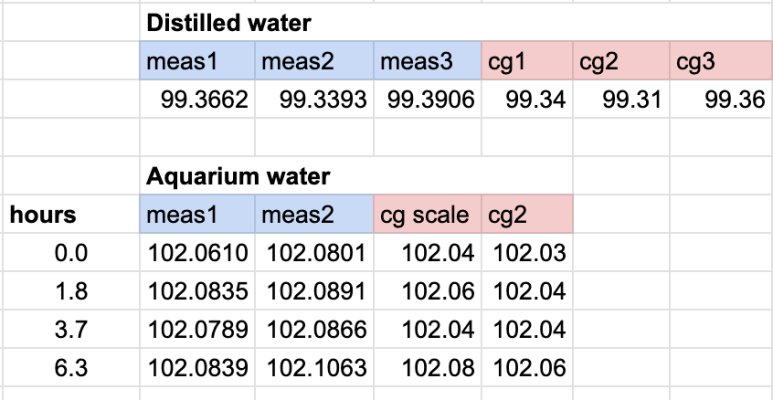
The measurements under blue header are with the fancy scale, and the ones under the red "cg" headers are the centigram 0.01g scale.
So here's what specific gravity looks like - simply taking the aquarium water mass measurements and dividing by the average of the distilled water mass measurements.

Interestingly, the cheap scale and the really good scale gave basically the same results, which means that the scale is probably not the limitation to the precision. It's how repeatable the water volume is.
So the water started around a SG of 1.0271-1.273 and finished at 1.0274-1.0276 which is a pretty nice result for a $20 piece of glassware.
Of course, If you just wanted to quantify evaporation, there are easier ways...like a ruler. Water level in the sump dropped 8mm.

I ran across a pycnometer for the first time a few months ago. And I eventually got one (two actually - 100mL and 10mL) to play with. In theory, a pycnometer and a good scale ought to give you a fundamental and highly trustworthy way to measure specific gravity.
It's not such an unusual an apparatus - it's just a special kind of volumetric flask made so that at a given temperature it can only possibly hold one volume of liquid. My 100mL pycnometer has a "certified volume" of 99.727mL at 20C.
So you can either work at that specific temp, and base your calculation off that certified volume, or - my preference is to just have tank water, distilled water and the pycnometer all at the same temp (water bath) and use specific gravity instead of density. For density I need exact volume - which means I need to know the temp and use the certified volume or measure it myself. For specific gravity, I just need a repeatable volume, distilled water and aquarium water at the same temp, and a scale. Since SG is fundamentally just a ratio, it cancels out a lot of measurement errors like if the flask volume is slightly off.
Here's how you fill it. You add liquid to the neck and then push the ground glass top stopper in place, it forces excess water up through a capillary and overflows, then you wipe off all excess. So the volume of liquid contained is pretty exact, and very repeatable.
Video of the filling process here
I wanted to see how good the precision was using this 100mL pycnometer, So I checked to see if it could measure the increase in salinity due to evaporation over 6 hours.
I took a few water samples over 6 hours, sealed them and put them in a tap water bath at room temp (24C) along with a sample of distilled water. after the last sample sat in the water bath for over an hour, I used the pycnometer to measure all the samples on a scale.
If the volume and temp are the same - specific gravity is simply the ratio (mass of sample / mass of pure water). So that's all I need.
I actually used two scales - the best one I have access to, goes to 0.0001g and a very not fancy centigram scale, goes to 0.01g.
Here's the measured masses in grams.

The measurements under blue header are with the fancy scale, and the ones under the red "cg" headers are the centigram 0.01g scale.
So here's what specific gravity looks like - simply taking the aquarium water mass measurements and dividing by the average of the distilled water mass measurements.
Interestingly, the cheap scale and the really good scale gave basically the same results, which means that the scale is probably not the limitation to the precision. It's how repeatable the water volume is.
So the water started around a SG of 1.0271-1.273 and finished at 1.0274-1.0276 which is a pretty nice result for a $20 piece of glassware.
Of course, If you just wanted to quantify evaporation, there are easier ways...like a ruler. Water level in the sump dropped 8mm.





















