Hey!
So I have a question about kH consumption in a fairly new tank. You can see my numbers below. Struggling with zero phosphate.
It’s stocked with 4 small torch/hammer drags in total.
I feel like the consumption of more than 1dkH every day is maybe too much? Can it be that the coralline algae started to grow without me seeing it? It’s ceramic rock.
I dose Tropic Marine All for reef based on the dkH number from the Reef Factory kH keeper plus.
It’s a innovative marine 20G.
Currently got bio balls and searched purigen in the back chambers. I also got a nano skimmer but I turned that off after seeing zero phosphate.
Thanks


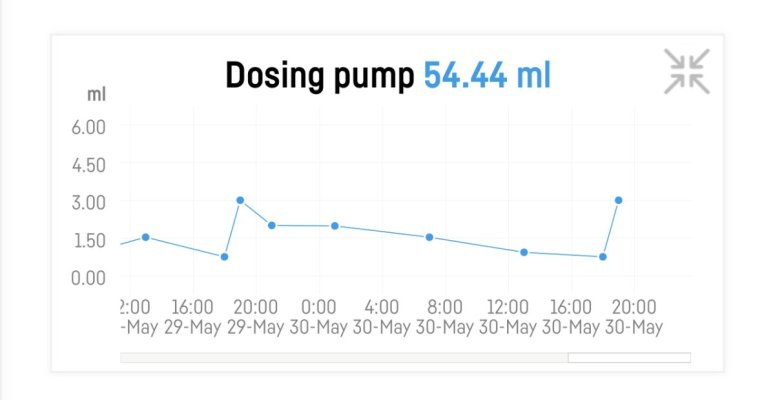
So I have a question about kH consumption in a fairly new tank. You can see my numbers below. Struggling with zero phosphate.
It’s stocked with 4 small torch/hammer drags in total.
I feel like the consumption of more than 1dkH every day is maybe too much? Can it be that the coralline algae started to grow without me seeing it? It’s ceramic rock.
I dose Tropic Marine All for reef based on the dkH number from the Reef Factory kH keeper plus.
It’s a innovative marine 20G.
Currently got bio balls and searched purigen in the back chambers. I also got a nano skimmer but I turned that off after seeing zero phosphate.
Thanks
















