I have made a similar reactor and am trying to dial it’s efficiency. I’m hovering around 7.9-8ph which isn’t what I was intending on hitting.
the reactor circulates the gas from the top of the chamber through a skimmer pump to mist the gas, some fresh is drawn in the balance out the vacuum created But mostly recirculated.
the gas is then drawn and fed into a secondary skimmer being used for gas exchange only no skimmate.
any ideas? I’m thinking the temps in garage may be effecting saturation of kalk? Or maybe not a large enough skimmer to exchange?

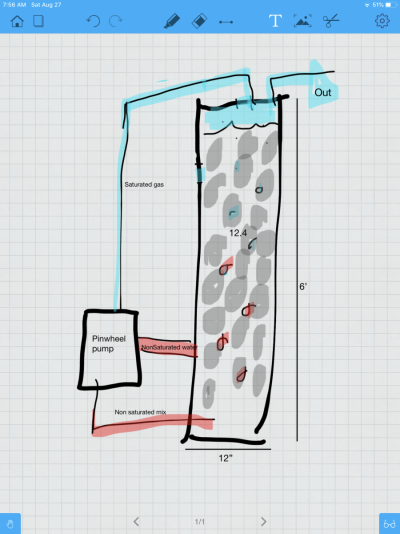
the reactor circulates the gas from the top of the chamber through a skimmer pump to mist the gas, some fresh is drawn in the balance out the vacuum created But mostly recirculated.
the gas is then drawn and fed into a secondary skimmer being used for gas exchange only no skimmate.
any ideas? I’m thinking the temps in garage may be effecting saturation of kalk? Or maybe not a large enough skimmer to exchange?


Last edited:

























