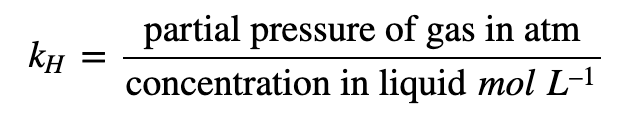- Joined
- May 22, 2016
- Messages
- 6,970
- Reaction score
- 10,747
I don't follow the freshwater.The pKa of ammonia will be different (higher, easier to make NH4+) in seawater than in fresh.
I smell (we'll assume) 50ppb in the air in the room.
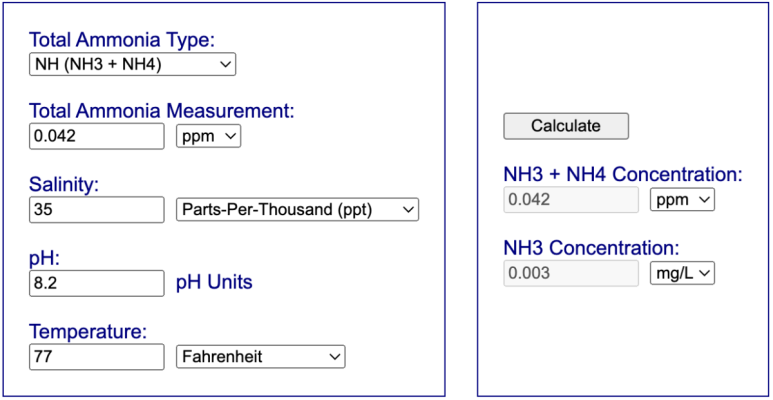
If the skimmer has equalized my saltwater tank (at pH 8.2, 77F) with that room air concentration, then I don't see the role for freshwater equilibrium.