How Much Two Part Do I Need?
I'm a two part doser for alkalinity and calcium (and magnesium), and have been repeatedly asked about how to figure out how much of each a new two-parter needs to dose to their tank. This subject has been thoroughly covered, so this article will predominantly be links to this information, and include my opinion and personal preferences.
What I'll review is:
A. Understanding Two Part Chemicals
B. The Need for Test Kits
C. A Video Summary on Two Part
D. So How Much Do I Need
A. Understanding Two Part Chemicals
The two parts are made up of an alkalinity part and a calcium part. The alkalinity part is commonly sodium bicarbonate (baking soda) or sodium carbonate (soda ash), while the calcium part is calcium chloride (associated with an amount of water). For alkalinity, I prefer soda ash for two reasons:
1. It's more soluble in water and therefore I can make a "strong" solution.
2. It pushes pH up oh so slightly more than baking soda.
The least expensive way that I've found to dose alkalinity is to make your own by simply purchasing baking soda and baking it in the oven for an hour. I purchase large bags from a club store for around 6 dollars for 13.5 pounds, which will make about 12 gallons of alkalinity solution. Simply spread 2 1/4 cups onto a baking sheet and bake at 350 for one hour. You have now converted baking soda to soda ash. Here's two batches ready to go into the oven:

Once they come out, mix with RO/DI water to make one gallon solutions. (With this example above, each cookie sheet has 2 1/4 cups of baking soda, enough to make 2 gallons of alkalinity solution in total.) Also note that once baked, the volume has been reduced to about 2 cups of soda ash on each sheet. You now have alkalinity solution to dose to your tank, and it cost you pennies.
When choosing a calcium part (when you're doing it by DIY), you need to have a rough estimate of the amount of bound water. For those using Dow Flakes (if you can find them), or BRS calcium, you need 2 1/2 cups per gallon of water, because it has a fair amount of water associated with the calcium chloride. For those using a more anhydrous form (less bound water), of calcium (such as Prestone Driveway Heat), you only need two cups per gallon. So for DIY'ers, take note of the water content of your calcium and adjust accordingly....between 2 to 2 1/2 cups per gallon.
Now, why do hard corals (and clams) need two part? The simplified chemistry is quite elegant. The corals take the carbonate from the alk part and the calcium from the calc part and combine the two to make calcium carbonate (the hard part of corals and clams), with the sodium and chloride part combining to make salt.
Na2CO3 + CaCl2 ----> CaCO3 + NaCl
Alk Part + Calc Part -----> Calcium Carbonate + Salt

Our own Dr. Randy Holmes Farley has written a number of articles on this subject, and suggest reading them to fully understand what is occurring with these two additives in our tanks:
The “How To” Guide to Reef Aquarium Chemistry for Beginners, Part 2: What Chemicals Must be Supplemented
Calcium and Alkalinity
A Simplified Guide to the Relationship Between Calcium, Alkalinity, Magnesium and pH
If you wish to delve further into the biochemistry of corals consuming calcium carbonate, Randy covers that in this article:
The Chemical and Biochemical Mechanisms of Calcification
And finally, if you wish to DIY your own two part, including calcium and magnesium, that is covered in this one:
An Improved Do-it-Yourself Two-Part Calcium and Alkalinity Supplement System
BRS uses Randy's recipe in the chemicals they sell, with no baking of baking soda needed because they sell soda ash directly. FYI, I bake my baking soda at 350 F instead of 300 mentioned in the above article.....doesn't hurt anything, and assures all is converted to the carbonate form. Note that extra time in the oven isn't critical either, so anything over an hour is fine.
B. The Need for Test Kits
If you're going to dose two part (or for that fact, any other means of maintaining alkalinity and calcium), you need a way of testing alkalinity, calcium and magnesium. I know you hate to do it, but I'm going to repeat, You Need To Test! The common choices are either Hanna checkers or wet chemistry test kits (titrations). For those not comfortable with wet chemistry, Hanna checkers are the way to go. Two errors I commonly see with Hanna's are:
1. Not cleaning the outside of the cuvette (vial) before putting it in the tester...finger prints need to be gone!
2. Putting the vial in the tester with liquid on the outside (which could ruin your tester).
So wipe it clean before going into the tester. You don't want to be putting this smudged up, wet vial into your Hanna Checker:

With wet chemistry methods, I am very familiar with API and Red Sea kits, both of which use titration as a means to determine both alkalinity and calcium. I am not aware of any alkalinity or calcium test kit that requires matching the color of the solution to a particular color on a card, typical of API nitrate, nitrite and ammonia kits, just to mention a few. If such color matching kits exist for alkalinity and calcium, I personally would not like them because I have a very difficult time matching the color in the vial to the color on the card. For some reason, when using these color matching kits, the color I get in the vial doesn't match any of the colors on the card. I should point out that I am colorblind, and that might very well be the reason I have difficulty with these kits. With the titration alkalinity and calcium kits—where I'm just looking for a color change—I have no problem.
So API and Red Sea (and others) are simple color changing titration kits, and my preference with these is the Red Sea Pro kit. This Pro kit includes all three test kits in one package; alkalinity, calcium and magnesium kits. This kit simply changes colors when the titration is done.

The common errors I see folks making with these kits are:
1. Not following instructions. Even though I've done these tests hundreds of times, I still pull out the summary card and follow the instructions explicitly. This is especially true with the magnesium test (from Red Sea), where you need to add five drops of a solution...see that little x5 on the card.

2. Thinking they need to match the color on the summary card to the color in the vial. The titration is over when it changes for one color to the other, where one more drop has no effect on the color. It's not like the test kits where you are matching the exact color on the card.
3. Reading the syringe wrong. With Red Sea, the syringe, when full is 1 ml. When you are done with the titration, the amount you put into the vial IS NOT THE READING ON THE SYRINGE. You need to take that reading on the syringe and subtract it from 1. So if the syringe says 0.4, the amount you used is 1 - 0.4 = 0.6 mls.
C. A Video Summary on Two Part
I'm including this video from BRS because I thought they did a good job of summarizing the two part system.
How To: Dosing Two Part in Your Reef Tank
D. So How Much Do I Need
Even though this has been covered in the linked articles and video above, I'm going to summarize my preferences in how to determine the amount of alkalinity and calcium I need to add to my tank on a daily basis.
INITIAL TESTING ----> ADJUST TO IDEAL LEVELS ---> DETERMINE CONSUMPTION ---> BEGIN DOSING
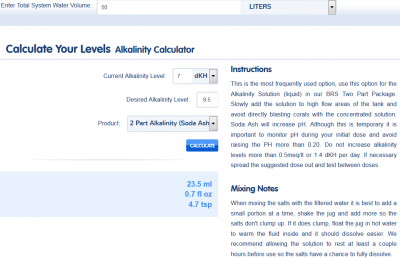
First thing is to test your alkalinity, calcium and magnesium levels and adjust them to where you would like them to be. Recommended levels are calcium around 400ppm, alkalinkity 7 - 11 dKH, and magnesium 1380 - 1400 ppm. When you do your original testing, use a reef calculator to determine how much you'll need to bring up your levels to where you'd wish them to be. I use the BRS calculator found HERE to figure out how much I'll need. Note that with calcium, you don't want to raise more than 50 ppm per day, and with alkalinity I like to raise no more than 1 dKH per day, until I'm where I want to be. When adding alkalinity and calcium, you want to add these in a high flow area, and not at the same time. If you add them at the same time, you'll get immediate precipitation of calcium carbonate. So separate the additions by a couple minutes to allow the solution to disperse.
Once you've gotten your numbers where you want them, test again about 24 hours later and note the drop. If you have an established tank with colonies of hard corals (and clams), you can use this number to determine your daily consumption, and again, using the calculator to determine how much you need to start dosing to maintain those levels. If you only have a few hard corals or just frags, you may wish to test again on day 2 and maybe day 3 to get a better idea of your daily consumption, and now use that number to determine dosing.
I would now recommend daily testing to make fine adjusts to the amounts you are dosing. Note with Magnesium, I do not dose daily. Dependent on the salt you are using, you may only need to dose weekly or monthly with magnesium. Continue daily testing and adjustments until you are confident in the way your tank is consuming alkalinity and calcium. Once stabilized, you can back off on testing to maybe weekly, or whatever time period you feel comfortable doing, knowing your tank numbers aren't moving.
Now sit back and watch your corals grow.
I'm a two part doser for alkalinity and calcium (and magnesium), and have been repeatedly asked about how to figure out how much of each a new two-parter needs to dose to their tank. This subject has been thoroughly covered, so this article will predominantly be links to this information, and include my opinion and personal preferences.
What I'll review is:
A. Understanding Two Part Chemicals
B. The Need for Test Kits
C. A Video Summary on Two Part
D. So How Much Do I Need
A. Understanding Two Part Chemicals
The two parts are made up of an alkalinity part and a calcium part. The alkalinity part is commonly sodium bicarbonate (baking soda) or sodium carbonate (soda ash), while the calcium part is calcium chloride (associated with an amount of water). For alkalinity, I prefer soda ash for two reasons:
1. It's more soluble in water and therefore I can make a "strong" solution.
2. It pushes pH up oh so slightly more than baking soda.
The least expensive way that I've found to dose alkalinity is to make your own by simply purchasing baking soda and baking it in the oven for an hour. I purchase large bags from a club store for around 6 dollars for 13.5 pounds, which will make about 12 gallons of alkalinity solution. Simply spread 2 1/4 cups onto a baking sheet and bake at 350 for one hour. You have now converted baking soda to soda ash. Here's two batches ready to go into the oven:
Once they come out, mix with RO/DI water to make one gallon solutions. (With this example above, each cookie sheet has 2 1/4 cups of baking soda, enough to make 2 gallons of alkalinity solution in total.) Also note that once baked, the volume has been reduced to about 2 cups of soda ash on each sheet. You now have alkalinity solution to dose to your tank, and it cost you pennies.
When choosing a calcium part (when you're doing it by DIY), you need to have a rough estimate of the amount of bound water. For those using Dow Flakes (if you can find them), or BRS calcium, you need 2 1/2 cups per gallon of water, because it has a fair amount of water associated with the calcium chloride. For those using a more anhydrous form (less bound water), of calcium (such as Prestone Driveway Heat), you only need two cups per gallon. So for DIY'ers, take note of the water content of your calcium and adjust accordingly....between 2 to 2 1/2 cups per gallon.
Now, why do hard corals (and clams) need two part? The simplified chemistry is quite elegant. The corals take the carbonate from the alk part and the calcium from the calc part and combine the two to make calcium carbonate (the hard part of corals and clams), with the sodium and chloride part combining to make salt.
Na2CO3 + CaCl2 ----> CaCO3 + NaCl
Alk Part + Calc Part -----> Calcium Carbonate + Salt
Our own Dr. Randy Holmes Farley has written a number of articles on this subject, and suggest reading them to fully understand what is occurring with these two additives in our tanks:
The “How To” Guide to Reef Aquarium Chemistry for Beginners, Part 2: What Chemicals Must be Supplemented
Calcium and Alkalinity
A Simplified Guide to the Relationship Between Calcium, Alkalinity, Magnesium and pH
If you wish to delve further into the biochemistry of corals consuming calcium carbonate, Randy covers that in this article:
The Chemical and Biochemical Mechanisms of Calcification
And finally, if you wish to DIY your own two part, including calcium and magnesium, that is covered in this one:
An Improved Do-it-Yourself Two-Part Calcium and Alkalinity Supplement System
BRS uses Randy's recipe in the chemicals they sell, with no baking of baking soda needed because they sell soda ash directly. FYI, I bake my baking soda at 350 F instead of 300 mentioned in the above article.....doesn't hurt anything, and assures all is converted to the carbonate form. Note that extra time in the oven isn't critical either, so anything over an hour is fine.
B. The Need for Test Kits
If you're going to dose two part (or for that fact, any other means of maintaining alkalinity and calcium), you need a way of testing alkalinity, calcium and magnesium. I know you hate to do it, but I'm going to repeat, You Need To Test! The common choices are either Hanna checkers or wet chemistry test kits (titrations). For those not comfortable with wet chemistry, Hanna checkers are the way to go. Two errors I commonly see with Hanna's are:
1. Not cleaning the outside of the cuvette (vial) before putting it in the tester...finger prints need to be gone!
2. Putting the vial in the tester with liquid on the outside (which could ruin your tester).
So wipe it clean before going into the tester. You don't want to be putting this smudged up, wet vial into your Hanna Checker:
With wet chemistry methods, I am very familiar with API and Red Sea kits, both of which use titration as a means to determine both alkalinity and calcium. I am not aware of any alkalinity or calcium test kit that requires matching the color of the solution to a particular color on a card, typical of API nitrate, nitrite and ammonia kits, just to mention a few. If such color matching kits exist for alkalinity and calcium, I personally would not like them because I have a very difficult time matching the color in the vial to the color on the card. For some reason, when using these color matching kits, the color I get in the vial doesn't match any of the colors on the card. I should point out that I am colorblind, and that might very well be the reason I have difficulty with these kits. With the titration alkalinity and calcium kits—where I'm just looking for a color change—I have no problem.
So API and Red Sea (and others) are simple color changing titration kits, and my preference with these is the Red Sea Pro kit. This Pro kit includes all three test kits in one package; alkalinity, calcium and magnesium kits. This kit simply changes colors when the titration is done.
The common errors I see folks making with these kits are:
1. Not following instructions. Even though I've done these tests hundreds of times, I still pull out the summary card and follow the instructions explicitly. This is especially true with the magnesium test (from Red Sea), where you need to add five drops of a solution...see that little x5 on the card.
2. Thinking they need to match the color on the summary card to the color in the vial. The titration is over when it changes for one color to the other, where one more drop has no effect on the color. It's not like the test kits where you are matching the exact color on the card.
3. Reading the syringe wrong. With Red Sea, the syringe, when full is 1 ml. When you are done with the titration, the amount you put into the vial IS NOT THE READING ON THE SYRINGE. You need to take that reading on the syringe and subtract it from 1. So if the syringe says 0.4, the amount you used is 1 - 0.4 = 0.6 mls.
C. A Video Summary on Two Part
I'm including this video from BRS because I thought they did a good job of summarizing the two part system.
How To: Dosing Two Part in Your Reef Tank
D. So How Much Do I Need
Even though this has been covered in the linked articles and video above, I'm going to summarize my preferences in how to determine the amount of alkalinity and calcium I need to add to my tank on a daily basis.
INITIAL TESTING ----> ADJUST TO IDEAL LEVELS ---> DETERMINE CONSUMPTION ---> BEGIN DOSING
First thing is to test your alkalinity, calcium and magnesium levels and adjust them to where you would like them to be. Recommended levels are calcium around 400ppm, alkalinkity 7 - 11 dKH, and magnesium 1380 - 1400 ppm. When you do your original testing, use a reef calculator to determine how much you'll need to bring up your levels to where you'd wish them to be. I use the BRS calculator found HERE to figure out how much I'll need. Note that with calcium, you don't want to raise more than 50 ppm per day, and with alkalinity I like to raise no more than 1 dKH per day, until I'm where I want to be. When adding alkalinity and calcium, you want to add these in a high flow area, and not at the same time. If you add them at the same time, you'll get immediate precipitation of calcium carbonate. So separate the additions by a couple minutes to allow the solution to disperse.
Once you've gotten your numbers where you want them, test again about 24 hours later and note the drop. If you have an established tank with colonies of hard corals (and clams), you can use this number to determine your daily consumption, and again, using the calculator to determine how much you need to start dosing to maintain those levels. If you only have a few hard corals or just frags, you may wish to test again on day 2 and maybe day 3 to get a better idea of your daily consumption, and now use that number to determine dosing.
I would now recommend daily testing to make fine adjusts to the amounts you are dosing. Note with Magnesium, I do not dose daily. Dependent on the salt you are using, you may only need to dose weekly or monthly with magnesium. Continue daily testing and adjustments until you are confident in the way your tank is consuming alkalinity and calcium. Once stabilized, you can back off on testing to maybe weekly, or whatever time period you feel comfortable doing, knowing your tank numbers aren't moving.
Now sit back and watch your corals grow.
Last edited: