I have a RS 525Xl 3 years running with Apex controller and Trident. I stopped water changes several months ago. I've only been dosing BRS sodium bicarbonate for Alk. I have a large chaeto ball I'm constantly trimming and maspect bio balls. I utilize Microbacter7 about everyb6 months. Along with Galaxy pods.
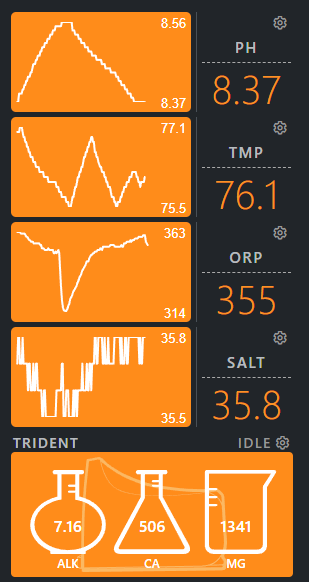
My trident regularly shows the Alk at 6.2 before adding SB then rises over 2 days of manually dosing to 7.8. Cal 350, Mag 1380 are both steady with little fluctuation and the salinity is steady at 35-36. Only have a few corals; zoas and blasto, but large amount of coraline algae.
I've been trying to figure out the chemistry and trying to stabilize the tank before adding additional corals. All of the livestock: fish, inverts and anemones are doing well.
I'm trying to figure out what the tank needs. I'm setting up 2 dosers for future needs but I don't want to inadvertently throw the chemistry off since the parameters have been steady other than Alk. I have not tested for phosphates for over a year.
I would like to start adding Euphyllia's again but don't want to watch them die off after a few months.
I would appreciate any fact based advise. I've already been given plenty of guesses. I don't believe water changes are the answer.
My trident regularly shows the Alk at 6.2 before adding SB then rises over 2 days of manually dosing to 7.8. Cal 350, Mag 1380 are both steady with little fluctuation and the salinity is steady at 35-36. Only have a few corals; zoas and blasto, but large amount of coraline algae.
I've been trying to figure out the chemistry and trying to stabilize the tank before adding additional corals. All of the livestock: fish, inverts and anemones are doing well.
I'm trying to figure out what the tank needs. I'm setting up 2 dosers for future needs but I don't want to inadvertently throw the chemistry off since the parameters have been steady other than Alk. I have not tested for phosphates for over a year.
I would like to start adding Euphyllia's again but don't want to watch them die off after a few months.
I would appreciate any fact based advise. I've already been given plenty of guesses. I don't believe water changes are the answer.