I agree and support Jim's results! When it comes from a chemistry perspective - and seeking accuracy of results, there is no disputing the equivalence point. Given it was obtained from a fully calibrated pH probe and accurately prepared seawater sample, which also contains the discrepancy of borate there is really nothing to contradict the methodology used here other than perhaps replication and the colour intensity from the 250 mL flask ;Happy. People need to understand the purpose of the indicator colour change is to assist in telling users when the reaction is complete; unlike a dye, a pH probe is far superior for accuracy and can pinpoint much stronger the true end point of the reaction.
It should be noted that people need to be aware multiple sources of error when comparing test results online, especially articles from many years ago, regardless of what parameter you choose. These errors can cause noticeable variations that need to be understood, which is why observing your own tank and having consistent & stable results is far superior than accurate results. Sources of error may include:
Thanks for posting the raw data @JimWelsh! It means I don't need to buy a $300 probe and can do some of my own extrapolation ;Pompus.
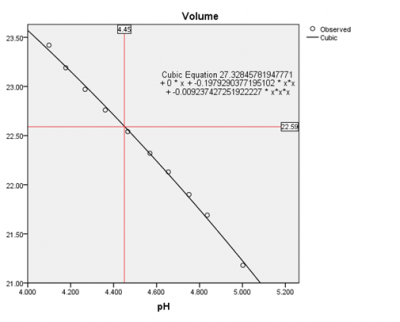
Based on the data provided, I've graphed the final equivalence zone to a cubic curve p <0.001, R2 = 0.998 (p = 6.09406173484311E-10 ;Woot). Note the pH values utilised were between 4-5, where the shift in pH values is greatest.Given the probe was calibrated I can infer a set pH end point value of 4.45 to provide the exact volume the test was completed using 0.02234N HCl. This value is indicated on the graph by the red lines - final volume at a pH of 4.45 was 22.59 mL. Provided I did the chemistry accurately, this equates to a alkalinity of 126.319 mg/L CaCO3; 2.522 mEq; 7.062 dkH.
Similarly using a linear regression model with a poorer fit, the calculated final volume was 22.57 mL equating to 126.19 mg/L CaCO3; 2.52 mEq; 7.055 dKh. The difference in volume between the two models was 0.023mL, which contributes to a difference of less than 0.129 mg/L CaCO3.
Now if we were to calculate the volume of acid needed to produce an end point at pH 4.2, the volume required is 23.15 mL. You can see the jump in volume from a pH difference of 0.25 units contributes to a large difference of 0.67 mL - an error of ~ +2.97% volume. This equates to an alkalinity of 129.437 mg/L CaCO3; 2.58 mEq; 7.236 dkH. The difference between the true end point illustrated by Jim and that overshot at a pH of 4.2 is 3.118 mg/L or ~ +2.47% error. This will therefore give a false reading of higher alkalinity than true.
Unfortunately I cannot provide a colour representation from the salifert at pH 4.2 - I assume this is where the bright pink colour is obtained with this specific test. Also, so it cannot be misinterpreted, I am a fan of salifert kits for consumer testing...
Finally, assuming user methodology that takes into account a pH probe that is out by 0.55 pH units and still rely solely on a pH value of 4.45 as the end point we would get a volume of 21.23 mL, Alk of 118.66 mg/L CaCO3; 2.369 mEq; 6.634 dKh - an error of ~ 6.06%. If you graphically calculate the equivalence point that is fine, but this assumes the lazy way of just relying on pH alone from a out of calibration probe.
Happy Reefing!

It should be noted that people need to be aware multiple sources of error when comparing test results online, especially articles from many years ago, regardless of what parameter you choose. These errors can cause noticeable variations that need to be understood, which is why observing your own tank and having consistent & stable results is far superior than accurate results. Sources of error may include:
- The test kit utilised (inbuilt margin of error by reagents chosen and test equipment provided)
- The methodology applied by the operator (us)
- The methodology recommended by the test manufacturer (as demonstrated here, salifert recommends a pink colour, where Jim has now shown the colour of the end point to be lavender)
- Age of kit used and storage procedures
- Interference of other chemicals within the aquarium tested
Thanks for posting the raw data @JimWelsh! It means I don't need to buy a $300 probe and can do some of my own extrapolation ;Pompus.
Based on the data provided, I've graphed the final equivalence zone to a cubic curve p <0.001, R2 = 0.998 (p = 6.09406173484311E-10 ;Woot). Note the pH values utilised were between 4-5, where the shift in pH values is greatest.Given the probe was calibrated I can infer a set pH end point value of 4.45 to provide the exact volume the test was completed using 0.02234N HCl. This value is indicated on the graph by the red lines - final volume at a pH of 4.45 was 22.59 mL. Provided I did the chemistry accurately, this equates to a alkalinity of 126.319 mg/L CaCO3; 2.522 mEq; 7.062 dkH.
Similarly using a linear regression model with a poorer fit, the calculated final volume was 22.57 mL equating to 126.19 mg/L CaCO3; 2.52 mEq; 7.055 dKh. The difference in volume between the two models was 0.023mL, which contributes to a difference of less than 0.129 mg/L CaCO3.
Now if we were to calculate the volume of acid needed to produce an end point at pH 4.2, the volume required is 23.15 mL. You can see the jump in volume from a pH difference of 0.25 units contributes to a large difference of 0.67 mL - an error of ~ +2.97% volume. This equates to an alkalinity of 129.437 mg/L CaCO3; 2.58 mEq; 7.236 dkH. The difference between the true end point illustrated by Jim and that overshot at a pH of 4.2 is 3.118 mg/L or ~ +2.47% error. This will therefore give a false reading of higher alkalinity than true.
Unfortunately I cannot provide a colour representation from the salifert at pH 4.2 - I assume this is where the bright pink colour is obtained with this specific test. Also, so it cannot be misinterpreted, I am a fan of salifert kits for consumer testing...
Finally, assuming user methodology that takes into account a pH probe that is out by 0.55 pH units and still rely solely on a pH value of 4.45 as the end point we would get a volume of 21.23 mL, Alk of 118.66 mg/L CaCO3; 2.369 mEq; 6.634 dKh - an error of ~ 6.06%. If you graphically calculate the equivalence point that is fine, but this assumes the lazy way of just relying on pH alone from a out of calibration probe.
Happy Reefing!

Last edited:
















