Last month I have someone. Frag rack. Today they have told me all their corals have died and that they have lost thousands worth.
they send me a picture of the rack with a small bit of rust, the size of a grain of rice.
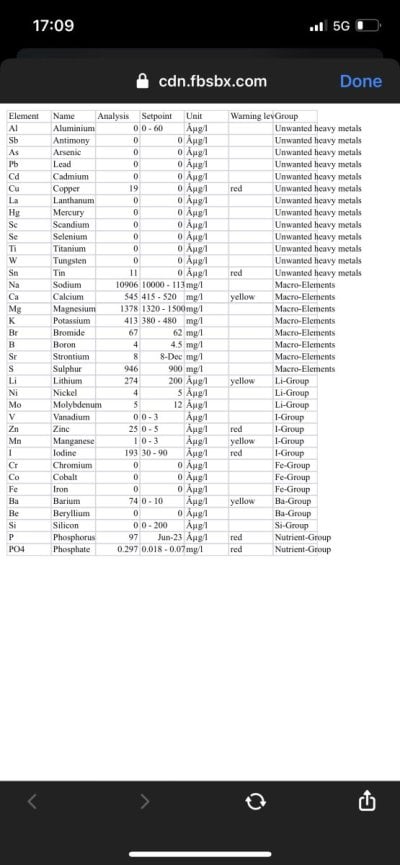
I asked for his icp which shows his po4 at 3! I said his corals dying is probably due to the high po4 and his iodine being very high.
I have attached his icp from triton and a picture of the rust.
would this amount of rust kill ao many corals in less than a month?

they send me a picture of the rack with a small bit of rust, the size of a grain of rice.
I asked for his icp which shows his po4 at 3! I said his corals dying is probably due to the high po4 and his iodine being very high.
I have attached his icp from triton and a picture of the rust.
would this amount of rust kill ao many corals in less than a month?
















