- Joined
- Sep 13, 2018
- Messages
- 360
- Reaction score
- 339
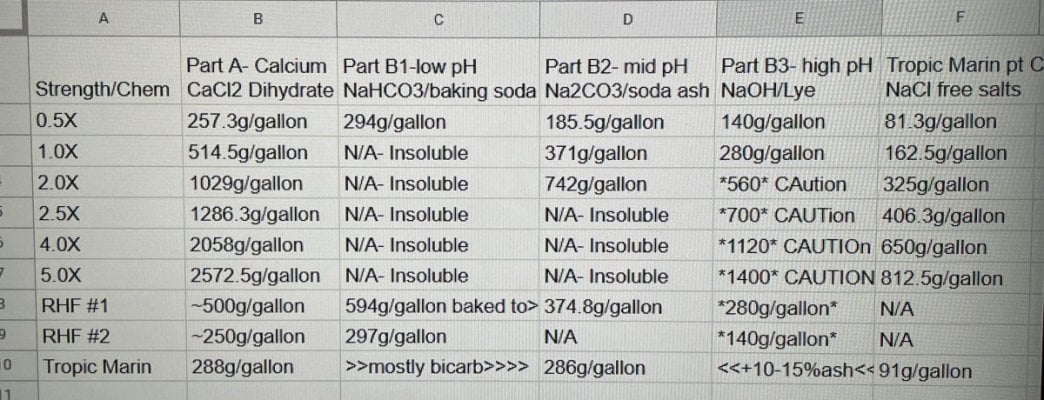
I’ve been using randy’s recipe for many years and added Tropic marin’s part C 3 years ago. I also began toggling the alk additive between bicarbonate, soda ash and lye to finely tune and maintain a high; stable pH. Tropic marin’s recipe is very diluted- I would have to mix, store and dose over 15 gallons of chems per month to maintain my 150g display’s demands. TM said their part C can’t be concentrated but I found otherwise- crystal clear and stable at nearly 9x their recipe.
Anyhow, i went about creating my new standard(1.0X) using RHF’s articles as inspiration. It appears he’s shooting for slightly over 3.5 moles calcium per gallon and double that in carbonate equivalent. His recipe assumes that dow flake is 78.5% pure CaCl2. Not many are using dowflake today but instead BRS’s CaCl2 dihydrate. If the dihydrate is pure, it should be 75.5% CaCl2. My 1.0X solutions aim for 3.50 moles Ca per gallon. I referenced TM’s CaCl2 tC ratio to get an accurate 1x number for part C.
tC ratio to get an accurate 1x number for part C.
Soda ash can be 2x concentrated and lye can be well over 10x. I definitely don’t recommend that. Instead; i prefer all my alk additives at 0.5x and Cal/ptC at 5X. This makes dosing easy. For every 100ml alk my tank demands I need to match it with 10.0ml A/C. Plus a 5g bucket of 5x lasts over 7 months
Hope this helps clarify things for people confused by all the different numbers.
Bonus tip: 2572.5g CaCl2 and 2660ml rodi should make exactly 1 gallon of solution. 812.5g ptC and 3420ml rodi should make exactly 1 gallon.
Anyhow, i went about creating my new standard(1.0X) using RHF’s articles as inspiration. It appears he’s shooting for slightly over 3.5 moles calcium per gallon and double that in carbonate equivalent. His recipe assumes that dow flake is 78.5% pure CaCl2. Not many are using dowflake today but instead BRS’s CaCl2 dihydrate. If the dihydrate is pure, it should be 75.5% CaCl2. My 1.0X solutions aim for 3.50 moles Ca per gallon. I referenced TM’s CaCl2
Soda ash can be 2x concentrated and lye can be well over 10x. I definitely don’t recommend that. Instead; i prefer all my alk additives at 0.5x and Cal/ptC at 5X. This makes dosing easy. For every 100ml alk my tank demands I need to match it with 10.0ml A/C. Plus a 5g bucket of 5x lasts over 7 months
Hope this helps clarify things for people confused by all the different numbers.
Bonus tip: 2572.5g CaCl2 and 2660ml rodi should make exactly 1 gallon of solution. 812.5g ptC and 3420ml rodi should make exactly 1 gallon.


















