Hi all,
Brand new user here, and praying this gets some traction.
I'm embarking on a sort of ridiculous project that it seems like this forum is uniquely poised to help with. I am a PhD student at Duke University studying the functional genomics of how calcifying marine invertebrates (think corals, shellfish, sea urchins, et cetera) repsond to climate change - essentially what genes or genetic variants most impact how these animals respond to ocean acidification. There is already a great wealth of data out there on this subject, but so far, no one has really managed to establish causal relationships or dig into what the genes that turn on or turn off in response to acidic seawater are doing, or why they're doing it. A huge part of that is technological limitations, and developing the technologies to do that is a passion of mine. I use the developing sea urchin embryo as a model organism. These little guys are fascinating. They develop from a fertilized egg into a swimming larvae with a complete calcium skeleton in a matter of hours - so speedy!
One thing I am trying to do is a type of genetic screen where we use CRISPR to make a pool of urchin embryos where basically every embryo has a different change introduced into its genome. Then we put half of those embryos in conditions that very closely approximate today's oceans, and another half in conditions that approximate the oceans of the near future. After allowing them to develop, we figure out which ones did the best, and which ones did the worst (we measure their fitness under these conditions). That data can be used for all sorts of cool things, including informing conservation approaches designed to preserve coral reefs for future generations.
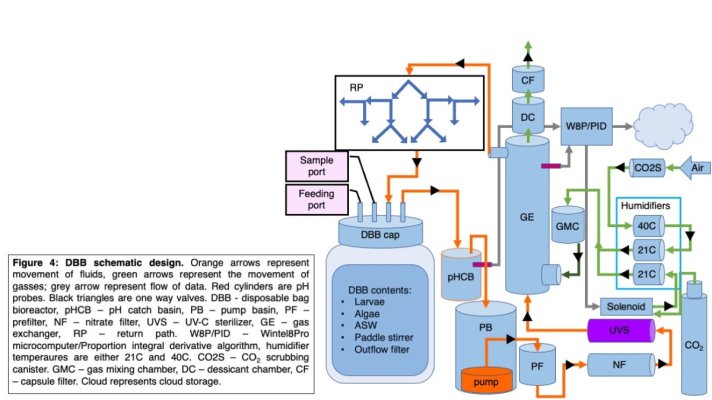
Heres my issue: everyone who works with these little critters tells me that these larvae can only be successfully raise at pretty low densities - around 1 larvae per mL of seawater. To actually do the screen, I need an absolutely massive number of embryos. We're talking about aquaculture setups on the scale of somewhere between 5000 and 100,000 liters. And because stewardship of taxpayer money for research is a high priority and because money dont grow on trees, I need to DIY these aquaria on the cheap. I'm thinking about borrowing an approach from the people trying to make lab-grown meat, and building something called a disposable bag bioreactor (DBB).
This is probably what you think it is: its a lot like a really fancy reef aquarium that got forced into a trash bag. My question to you all is this: if you had to take a really precious reef aquarium and exchange the actual tank for a trash bag, how would you do it?
I have some ideas and have been researching this for nearly a year, but after reading through posts here I am coming to realize that when it comes to managing complex aquaria, you all have vastly more expertise than I think I could ever hope to gain. I'm interested in hearing two things - responses to the seemingly silly but totally serious trash bag question above, and responses to some specific things I'm trying to work though that I'll list below.
Thank you all so much in advance. If you don't terribly mind, tag friends on here that you think might be interested in this problem - the more reach this can get the more successful I will likely be in my research.
Sincerely,
Carl
Specific issues I am maybe stumped by:
Automated control of pH - I plan to bubble CO2 through the bags and use a PID controller and solenoid to maintain pH. But this necessitates pH measurement. How can I measure the pH (and other parameters like temperature, alkalinity, nitrate levels, et cetera) of dozens of 125L cultures in sealed plastic bags without having to get dozens of pH meters? Are there other parameters I should be measuring - if it impacts the health of corals in your hands, it's probably important here.
Filtration rates - in small cultures I have to change the water every 2-3 days. In a big culture like this, thats really not doable. So I think I have to filter the water. When I filter urchins embryos, I have to be really careful about flow rates - if I pull too hard, they all get stuck to the mesh that keeps them out of the pump and they get shredded by the shear forces. What's the lowest rate of filtration you've had success with in a tank that doesn't have fish (by which I mean, where nitrate levels aren't the limiting factor)?
Lastly, if anyone has any advice about how to keep Rhodomonas lens cultures from crashing, or more hardy marine microalgae that are a good source of lipids, I am all ears!
Brand new user here, and praying this gets some traction.
I'm embarking on a sort of ridiculous project that it seems like this forum is uniquely poised to help with. I am a PhD student at Duke University studying the functional genomics of how calcifying marine invertebrates (think corals, shellfish, sea urchins, et cetera) repsond to climate change - essentially what genes or genetic variants most impact how these animals respond to ocean acidification. There is already a great wealth of data out there on this subject, but so far, no one has really managed to establish causal relationships or dig into what the genes that turn on or turn off in response to acidic seawater are doing, or why they're doing it. A huge part of that is technological limitations, and developing the technologies to do that is a passion of mine. I use the developing sea urchin embryo as a model organism. These little guys are fascinating. They develop from a fertilized egg into a swimming larvae with a complete calcium skeleton in a matter of hours - so speedy!
One thing I am trying to do is a type of genetic screen where we use CRISPR to make a pool of urchin embryos where basically every embryo has a different change introduced into its genome. Then we put half of those embryos in conditions that very closely approximate today's oceans, and another half in conditions that approximate the oceans of the near future. After allowing them to develop, we figure out which ones did the best, and which ones did the worst (we measure their fitness under these conditions). That data can be used for all sorts of cool things, including informing conservation approaches designed to preserve coral reefs for future generations.
Heres my issue: everyone who works with these little critters tells me that these larvae can only be successfully raise at pretty low densities - around 1 larvae per mL of seawater. To actually do the screen, I need an absolutely massive number of embryos. We're talking about aquaculture setups on the scale of somewhere between 5000 and 100,000 liters. And because stewardship of taxpayer money for research is a high priority and because money dont grow on trees, I need to DIY these aquaria on the cheap. I'm thinking about borrowing an approach from the people trying to make lab-grown meat, and building something called a disposable bag bioreactor (DBB).
This is probably what you think it is: its a lot like a really fancy reef aquarium that got forced into a trash bag. My question to you all is this: if you had to take a really precious reef aquarium and exchange the actual tank for a trash bag, how would you do it?
I have some ideas and have been researching this for nearly a year, but after reading through posts here I am coming to realize that when it comes to managing complex aquaria, you all have vastly more expertise than I think I could ever hope to gain. I'm interested in hearing two things - responses to the seemingly silly but totally serious trash bag question above, and responses to some specific things I'm trying to work though that I'll list below.
Thank you all so much in advance. If you don't terribly mind, tag friends on here that you think might be interested in this problem - the more reach this can get the more successful I will likely be in my research.
Sincerely,
Carl
Specific issues I am maybe stumped by:
Automated control of pH - I plan to bubble CO2 through the bags and use a PID controller and solenoid to maintain pH. But this necessitates pH measurement. How can I measure the pH (and other parameters like temperature, alkalinity, nitrate levels, et cetera) of dozens of 125L cultures in sealed plastic bags without having to get dozens of pH meters? Are there other parameters I should be measuring - if it impacts the health of corals in your hands, it's probably important here.
Filtration rates - in small cultures I have to change the water every 2-3 days. In a big culture like this, thats really not doable. So I think I have to filter the water. When I filter urchins embryos, I have to be really careful about flow rates - if I pull too hard, they all get stuck to the mesh that keeps them out of the pump and they get shredded by the shear forces. What's the lowest rate of filtration you've had success with in a tank that doesn't have fish (by which I mean, where nitrate levels aren't the limiting factor)?
Lastly, if anyone has any advice about how to keep Rhodomonas lens cultures from crashing, or more hardy marine microalgae that are a good source of lipids, I am all ears!