Hello everybody!
I need your help.
I have had some problems with diatom algae. I had a recent ICP and the silica was very high (1007mg/l). My RODI has silica at 0.0 so I suspect that the silica was coming from the ceramics, but I already removed them.
Until now I was trying to control the nutrients with a carboo source, but I decided to put chaetomorpha in my refuge. The chaeto is only 1 week old and I notice that it has some white tips.
The big issue now is that I can't get PO4 readings on the Hanna checker, even dosing 0.16ppm per day. I dose Nyos phosphate +.
Do you think I should continue dosing this amount of PO4 per day? Do you think the diatoms are absorbing all the incoming PO4? or the rock?
Is it possible the chaeto is turning white due to the low PO4, even at 0.16ppm per day?
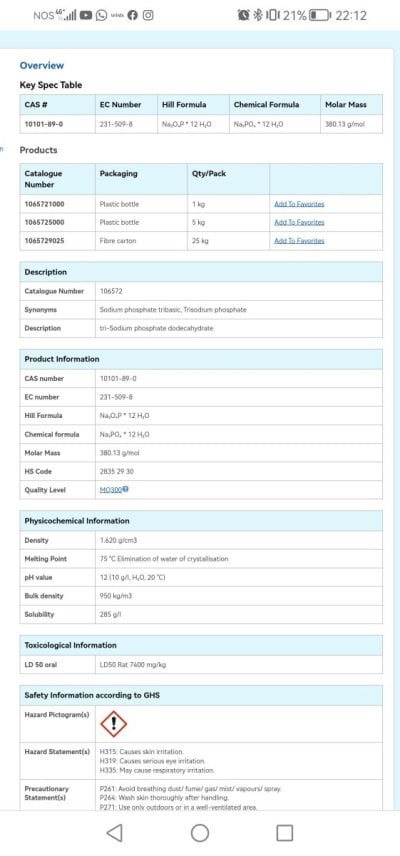
Parameters:
Po4 - 0.00 (Hanna Checker) / 0.018 (ICP)
No3 - 30 ppm
Magnesio - 1400
Cálcio - 424
Salinity - 1026
Silica - 1.007 mg/l
Aluminium - 170 ug/l
Thanks and sorry for my english.


I need your help.
I have had some problems with diatom algae. I had a recent ICP and the silica was very high (1007mg/l). My RODI has silica at 0.0 so I suspect that the silica was coming from the ceramics, but I already removed them.
Until now I was trying to control the nutrients with a carboo source, but I decided to put chaetomorpha in my refuge. The chaeto is only 1 week old and I notice that it has some white tips.
The big issue now is that I can't get PO4 readings on the Hanna checker, even dosing 0.16ppm per day. I dose Nyos phosphate +.
Do you think I should continue dosing this amount of PO4 per day? Do you think the diatoms are absorbing all the incoming PO4? or the rock?
Is it possible the chaeto is turning white due to the low PO4, even at 0.16ppm per day?
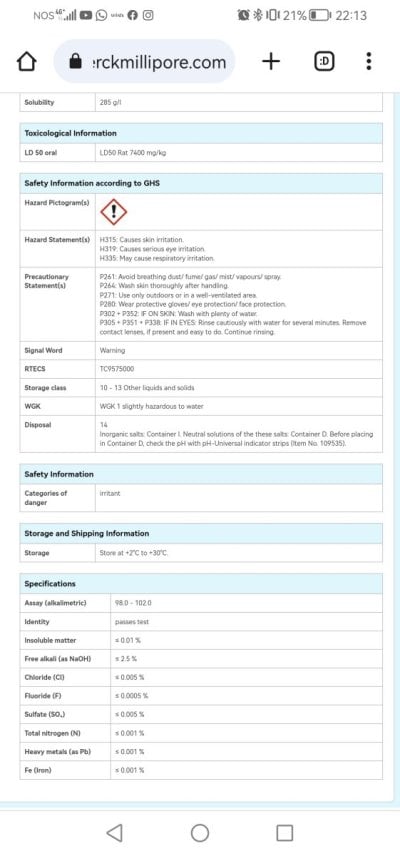
Parameters:
Po4 - 0.00 (Hanna Checker) / 0.018 (ICP)
No3 - 30 ppm
Magnesio - 1400
Cálcio - 424
Salinity - 1026
Silica - 1.007 mg/l
Aluminium - 170 ug/l
Thanks and sorry for my english.