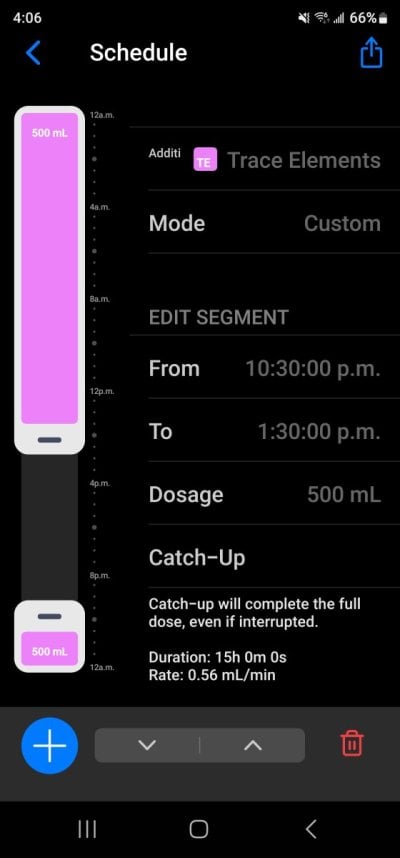
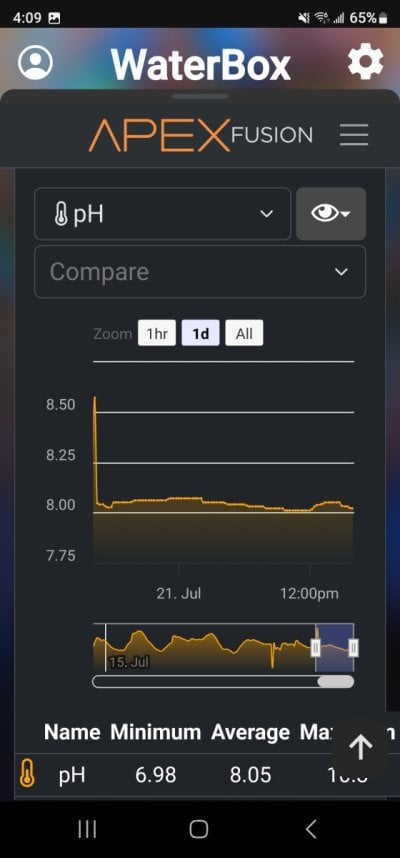
So apologies if this has been answered before numerous times or is just common knowledge but I am thinking to use kalkwasser on my new build (Elos 70 60g total) I’m currently cycling and was aiming to use a 5-10L dosing vessel with a tight fitting lid, originally I was planning to get a stirrer or something like that then realised it’s not entirely necessary.
My questions are:
Should I not even think of using this like 2-part dosing until I have some corals to consume the all etc, and just use a CO2 scrubber for the skimmer?
So many videos talk about, add your teaspoons and water, “set your dose and that’s all there is to it” but a noob like me is thinking, ok you said don’t dose the slurry, but when it’s time to refill that container and in my case it’ll be more often given the size container I’m going for, what do you do with the slurry after you’re done? Do you refill with rodi and not add more kalk or do you wash the container out and start again, or none of the above
I guess I have seen people talk about the importance of ph testing the saturated liquid to know you have the right mix, but just haven’t seen anything talk about the existing media.
Also one last question, am I guaranteed to have chalking problems on all my gear when dosing kalk slowly? Just thinking if the benefits outweigh the chore of maintenance.
Thanks in advance!
My questions are:
Should I not even think of using this like 2-part dosing until I have some corals to consume the all etc, and just use a CO2 scrubber for the skimmer?
So many videos talk about, add your teaspoons and water, “set your dose and that’s all there is to it” but a noob like me is thinking, ok you said don’t dose the slurry, but when it’s time to refill that container and in my case it’ll be more often given the size container I’m going for, what do you do with the slurry after you’re done? Do you refill with rodi and not add more kalk or do you wash the container out and start again, or none of the above
I guess I have seen people talk about the importance of ph testing the saturated liquid to know you have the right mix, but just haven’t seen anything talk about the existing media.
Also one last question, am I guaranteed to have chalking problems on all my gear when dosing kalk slowly? Just thinking if the benefits outweigh the chore of maintenance.
Thanks in advance!