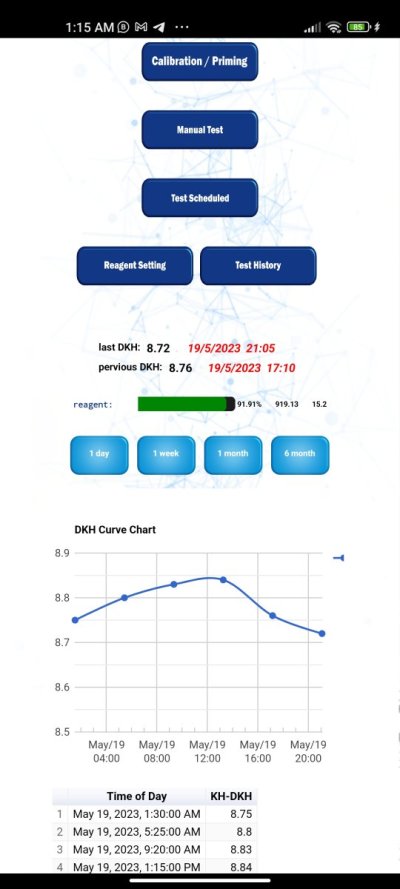
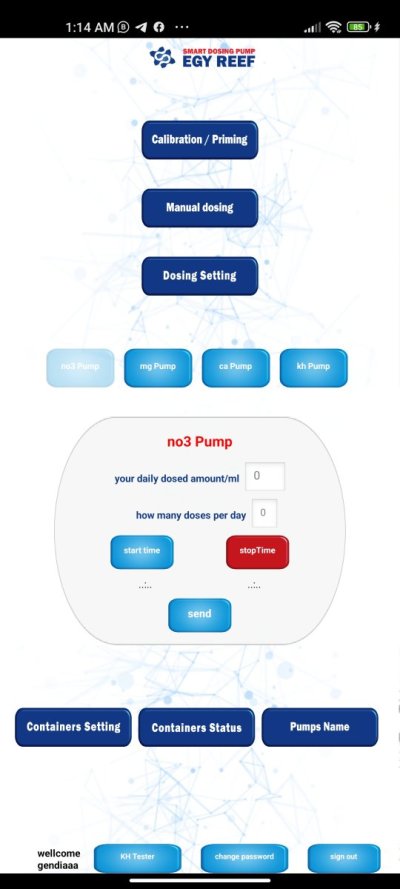
I really want to make my DIY nitrate test was looking for a lot of methods all depends in N.E.D ethyelenediamine dihydrochloride
If anyone please know good method for high range nitrate this gonna be helpful
If anyone please know good method for high range nitrate this gonna be helpful