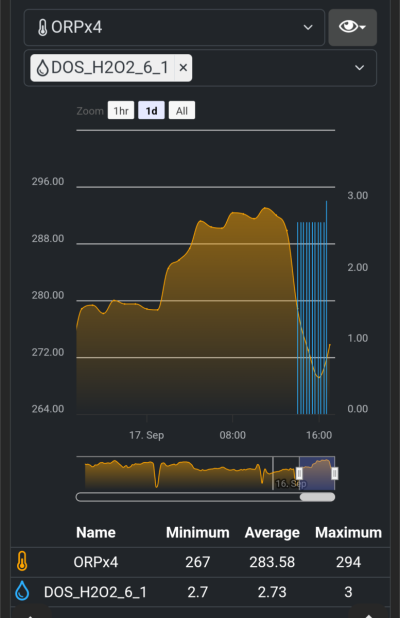
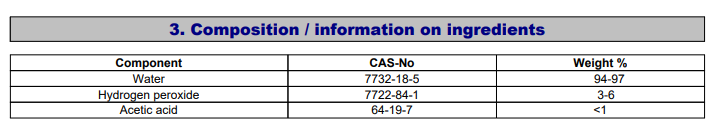
To be clear, this is about longer term oxidizer dosing (with dose measurement and monitoring), not topical applications or dips for algae or pest treatment.
After watching a fair amount of BRS and other reef content on YouTube discussing the benefits of dosing ozone to clarify water and reduce risks of infectious bateria, I started to wonder about using hydrogen peroxide to similar effect (i know i am not the first). Naturally this became a rabbit hole of reading through threads and some literature with many emotions and anecdotes but perhaps not much science (excepting Randy as referenced below). There are several threads claiming that 10ml of 3-percent (or other small amount) hydrogen peroxide crashed a tank and others claiming that hydrogen peroxide dosing is dangerous, or pointless (maybe...). I wanted to gather any research that people have done on long term hydrogen peroxide dosing to compile a best practices for folks who are similarly curious and help answer some questions about when using an oxidizer to raise ORP might be helpful, what are the benefits or downsides of hydrogen peroxide relative to ozone, and when should we bother with hydrogen peroxide to address specific issues. If this exists, I didn't find it...but if it does, please show me the way?
According to this great article by Mr. Randy Holmes, (https://reefkeeping.com/issues/2003-12/rhf/feature/) there is questionable benefit to attempting to manage ORP with oxidizers in place of seeking to manage the underlying problems that might depress ORP; however, many folks have persisted and insist that running ozone or other oxidation methods can help manage nuisance algaes and other bacterial infestations and will help with water clarity.
The issue that got me going on this was the relative hazards related to dosing ozone:
1) Ozone is harmful to indoor air and needs to be quenched to avoid poluting your air quality
2) Ozone gas will burn many materials including the seals and tubing in a skimmer
3) Ozone is a stong oxidizer and mistakes with dosing can rocket ORP and harm the inhabitants
4) Ozone generators are expensive, are damaged by air moisture, and need maintenance and replacement.
And the potential relative benefits of hydrogen peroxide:
1) Has similar oxidation properties as ozone (albeit oxidation strength depends on concentration)
2) Does not release a harmful gas into indoor air
3) At 3-percent concentration it is much more difficult to overdose (but also a weaker oxidizer)
4) Unlikely to burn equipment except at very high concentration
5) Injection under UV lighting may enhance photolysis allowing for a stronger oxidation reaction with a weaker oxidant and a smaller UV light.
6) A gallon of 3% peroxide at Costco is usually <$10 making this a good value especially for smaller tanks .
Some questions...
-Why do people tend to prefer ozone over hydrogen peroxide?
-Are people adding peroxide to the intake of UV sterlizers or under strong UV lighting? Why dose at night instead of peak light cycle
-What are the measurable differences in using small high concentration doses of ozone versus a lower strength hydrogen peroxide dose to elevate ORP or address nuisance outbreaks in a reef aquarium?
I am a professional groundwater hydrologist with some experience in water treatment, but not a chemist or water treatment expert. With work and family balances, I am a frequently neglectful reefer and I am often looking for approaches that can give me some added security between extended water changes or those odd algae outbreaks that I am slow to address.
After watching a fair amount of BRS and other reef content on YouTube discussing the benefits of dosing ozone to clarify water and reduce risks of infectious bateria, I started to wonder about using hydrogen peroxide to similar effect (i know i am not the first). Naturally this became a rabbit hole of reading through threads and some literature with many emotions and anecdotes but perhaps not much science (excepting Randy as referenced below). There are several threads claiming that 10ml of 3-percent (or other small amount) hydrogen peroxide crashed a tank and others claiming that hydrogen peroxide dosing is dangerous, or pointless (maybe...). I wanted to gather any research that people have done on long term hydrogen peroxide dosing to compile a best practices for folks who are similarly curious and help answer some questions about when using an oxidizer to raise ORP might be helpful, what are the benefits or downsides of hydrogen peroxide relative to ozone, and when should we bother with hydrogen peroxide to address specific issues. If this exists, I didn't find it...but if it does, please show me the way?
According to this great article by Mr. Randy Holmes, (https://reefkeeping.com/issues/2003-12/rhf/feature/) there is questionable benefit to attempting to manage ORP with oxidizers in place of seeking to manage the underlying problems that might depress ORP; however, many folks have persisted and insist that running ozone or other oxidation methods can help manage nuisance algaes and other bacterial infestations and will help with water clarity.
The issue that got me going on this was the relative hazards related to dosing ozone:
1) Ozone is harmful to indoor air and needs to be quenched to avoid poluting your air quality
2) Ozone gas will burn many materials including the seals and tubing in a skimmer
3) Ozone is a stong oxidizer and mistakes with dosing can rocket ORP and harm the inhabitants
4) Ozone generators are expensive, are damaged by air moisture, and need maintenance and replacement.
And the potential relative benefits of hydrogen peroxide:
1) Has similar oxidation properties as ozone (albeit oxidation strength depends on concentration)
2) Does not release a harmful gas into indoor air
3) At 3-percent concentration it is much more difficult to overdose (but also a weaker oxidizer)
4) Unlikely to burn equipment except at very high concentration
5) Injection under UV lighting may enhance photolysis allowing for a stronger oxidation reaction with a weaker oxidant and a smaller UV light.
6) A gallon of 3% peroxide at Costco is usually <$10 making this a good value especially for smaller tanks .
Some questions...
-Why do people tend to prefer ozone over hydrogen peroxide?
-Are people adding peroxide to the intake of UV sterlizers or under strong UV lighting? Why dose at night instead of peak light cycle
-What are the measurable differences in using small high concentration doses of ozone versus a lower strength hydrogen peroxide dose to elevate ORP or address nuisance outbreaks in a reef aquarium?
I am a professional groundwater hydrologist with some experience in water treatment, but not a chemist or water treatment expert. With work and family balances, I am a frequently neglectful reefer and I am often looking for approaches that can give me some added security between extended water changes or those odd algae outbreaks that I am slow to address.