- Joined
- May 17, 2020
- Messages
- 185
- Reaction score
- 245
How high, is too high - for pH?
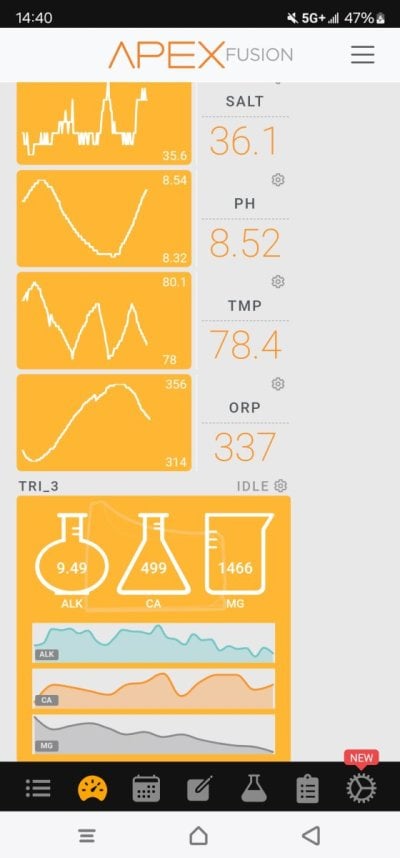
Over the years I've read...the higher the pH the better. Heard rumors of some high end reefers pushing 9+ - doing a lot obviously to achieve this.
Now me, I'm currently pulling fresh air from my skimmer & dripping, 6mls of kalkwasser per minute (or adding little over 8.6L a day). Basically got my kalk replacing evaporation, at this point. This is giving my a nice pH boost. Corals - doing fantastic, never seen better coral growth!
Alk - Calc and Mag generally run elevated - dosing minimal, due to amount kalkwasser. Don't need to dose as much 2 part.
BUT, I started thinking....is running elevated pH in a reef tank, a bad thing? I mean - the higher the pH, the more alkaline the water, no?
Right now I'm fallow (velvet outbreak) - but restocking in a few weeks. Now - when I did have fish, they were all happy, healthy and fat (well - till I got velvet). But I'm just curious....if the elevated pH - will cause me issues, moving fwd?
Some readings indicated high pH = stress and what not...but doesn't talk about HOW high of pH.
Thoughts? Or am I just overthinking...
Over the years I've read...the higher the pH the better. Heard rumors of some high end reefers pushing 9+ - doing a lot obviously to achieve this.
Now me, I'm currently pulling fresh air from my skimmer & dripping, 6mls of kalkwasser per minute (or adding little over 8.6L a day). Basically got my kalk replacing evaporation, at this point. This is giving my a nice pH boost. Corals - doing fantastic, never seen better coral growth!
Alk - Calc and Mag generally run elevated - dosing minimal, due to amount kalkwasser. Don't need to dose as much 2 part.
BUT, I started thinking....is running elevated pH in a reef tank, a bad thing? I mean - the higher the pH, the more alkaline the water, no?
Right now I'm fallow (velvet outbreak) - but restocking in a few weeks. Now - when I did have fish, they were all happy, healthy and fat (well - till I got velvet). But I'm just curious....if the elevated pH - will cause me issues, moving fwd?
Some readings indicated high pH = stress and what not...but doesn't talk about HOW high of pH.
Thoughts? Or am I just overthinking...





















