Ammo Lock claims to measure ammonia reduction with API test kits - at least according to the bottle I have. I could take a picture - but it's not that big of a deal. Thoughts? I guess I can check if I still have some Dr Tims available to make a test - but - it goes against the "R2R gold standard" that nothing lowers free ammonia. @taricha - @Dan_P P
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.

Note: This feature currently requires accessing the site using the built-in Safari browser.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
For the corporate conspiracy theorists - Ammo-Lock
- Thread starter MnFish1
- Start date
nereefpat
5000 Club Member
- Review score
- +0 /0 /-0
Reef Squad
R2R Supporter
R2R Excellence Award
Fish Medic
- Joined
- Apr 20, 2018
- Messages
- 7,127
- Reaction score
- 7,903
- Review score
- +0 /0 /-0
- Location
- Central Nebraska
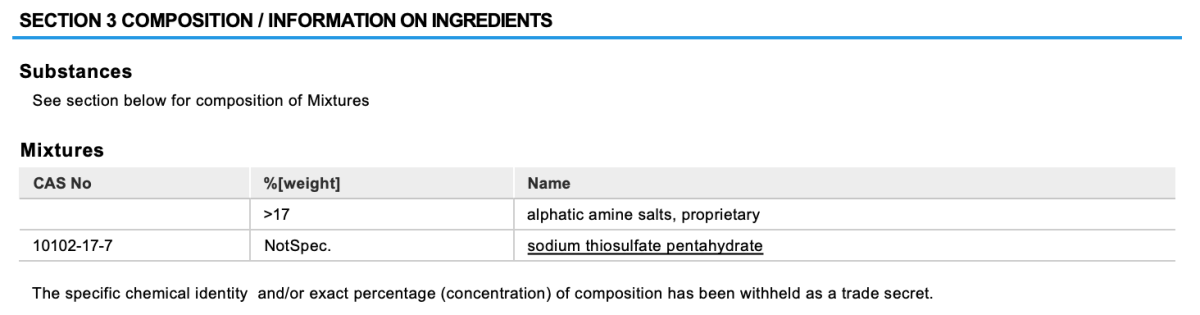
According to the SDS: https://apifishcare.com/pdfs/products-us/ammo-lock/ammo-lock-safety-data-sheet.pdf
It's sodium thiosulfate (not surprising, same chlorine neutralizer that's in other stuff)
+ some proprietary blend of "alphatic" amine salts (typo for aliphatic?).
It's sodium thiosulfate (not surprising, same chlorine neutralizer that's in other stuff)
+ some proprietary blend of "alphatic" amine salts (typo for aliphatic?).
Last I remember, Ammo-lock lists thiosulphate as an ingredient.
Thus the dechlorinating effect of thiosulphate would interfere with the chlorine in reagent 2 of the API Total ammonia test causing a lower measurement.
any significant amount of dechlorinator / reducing agent is going to lower the final color of the total ammonia tests to some degree.
So reduction of ammonia from ammo-lock is actually not verifiable with API kits, only interference as usual...
Edit: @nereefpat posted the SDS. It lists an undisclosed amount of thiosulphate.
Thus the dechlorinating effect of thiosulphate would interfere with the chlorine in reagent 2 of the API Total ammonia test causing a lower measurement.
any significant amount of dechlorinator / reducing agent is going to lower the final color of the total ammonia tests to some degree.
So reduction of ammonia from ammo-lock is actually not verifiable with API kits, only interference as usual...
Edit: @nereefpat posted the SDS. It lists an undisclosed amount of thiosulphate.

To be fair, their patent says it's the amines that bind the ammonia...It's sodium thiosulfate (not surprising, same chlorine neutralizer that's in other stuff)
+ some proprietary blend of "alphatic" amine salts (typo for aliphatic?).
But then they add thiosulphate so such an effect can't actually be verified with hobby kits.
nereefpat
5000 Club Member
- Review score
- +0 /0 /-0
Reef Squad
R2R Supporter
R2R Excellence Award
Fish Medic
- Joined
- Apr 20, 2018
- Messages
- 7,127
- Reaction score
- 7,903
- Review score
- +0 /0 /-0
- Location
- Central Nebraska
If the amines bind the ammonia, then why add thiosulfate? Rhetorical, of course.To be fair, their patent says it's the amines that bind the ammonia...
But then they add thiosulphate so such an effect can't actually be verified with hobby kits.
It's my understanding that there isn't anything that can work in saltwater. I wonder if would even work in freshwater? Maybe you'd know?
Is this the patent that includes ethanolamine as one of the claimed chemical structure?To be fair, their patent says it's the amines that bind the ammonia...
But then they add thiosulphate so such an effect can't actually be verified with hobby kits.
Similar threads
- Replies
- 3
- Views
- 315
- Replies
- 20
- Views
- 443
- Replies
- 22
- Views
- 1,015














