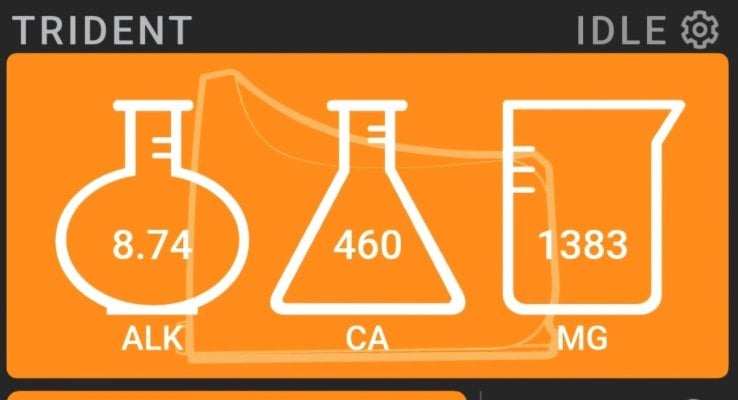
Hi all,
Question. Can I add Alk without raising PH using a DIY bicarbonate solution and use AFR as my Calcium and Mag dose?
I’m currently using BA Alkalin 8.3-P to keep my Alk at 8.3 Dkh and I use AFR o supplement the cal and mag losses. I hand dose twice daily. However, my PH swings into the high 8.4s at night. Brightwell claims their KH buffer won’t elevate Ph beyond a stable 8.3, but my experience does not support that claim.
My tank is a 80g total volume running with about 70 gallons of actual water volume with rock and sand. I have 3 smaller sized torches, a large colony of branching hammer, two smaller hammers, 2 favia frags, birdsnest, small zoa garden, And a couple monti cap frags. All in all, a light coral load right now until things get bigger. My daily alk consumption is now around the .20 Dkh, however my calcium consumption is less than 2 ppm and mag is around 5 ppm daily.
My NO3 flexes between 15-20 ppm but otherwise stable in that range for about a year. I do water changes of about 15% every 2 weeks but slowly moving toward a monthly WC of the same volume
Can I mix a stand alone bicarbonate solution to replace the Alkalin 8.3-P? If so, is there a quick recipe?
I’m trying to avoid doing a standard 3 part recipe and using the above as a hybrid as it’s a bit more manageable for my circumstance. I also want to continue hand dosing in the morning and at night.
I apologize if all the needed info isn’t there, but hoping there’s enough to start a conversation. Also, I’m just passing my first year in reefing, so I’m not an expert or have above average knowledge, but I’m a quick learner.
Thanks in advance.
Zig.
Question. Can I add Alk without raising PH using a DIY bicarbonate solution and use AFR as my Calcium and Mag dose?
I’m currently using BA Alkalin 8.3-P to keep my Alk at 8.3 Dkh and I use AFR o supplement the cal and mag losses. I hand dose twice daily. However, my PH swings into the high 8.4s at night. Brightwell claims their KH buffer won’t elevate Ph beyond a stable 8.3, but my experience does not support that claim.
My tank is a 80g total volume running with about 70 gallons of actual water volume with rock and sand. I have 3 smaller sized torches, a large colony of branching hammer, two smaller hammers, 2 favia frags, birdsnest, small zoa garden, And a couple monti cap frags. All in all, a light coral load right now until things get bigger. My daily alk consumption is now around the .20 Dkh, however my calcium consumption is less than 2 ppm and mag is around 5 ppm daily.
My NO3 flexes between 15-20 ppm but otherwise stable in that range for about a year. I do water changes of about 15% every 2 weeks but slowly moving toward a monthly WC of the same volume
Can I mix a stand alone bicarbonate solution to replace the Alkalin 8.3-P? If so, is there a quick recipe?
I’m trying to avoid doing a standard 3 part recipe and using the above as a hybrid as it’s a bit more manageable for my circumstance. I also want to continue hand dosing in the morning and at night.
I apologize if all the needed info isn’t there, but hoping there’s enough to start a conversation. Also, I’m just passing my first year in reefing, so I’m not an expert or have above average knowledge, but I’m a quick learner.
Thanks in advance.
Zig.