- Joined
- Aug 1, 2021
- Messages
- 166
- Reaction score
- 151
- Review score
- +0 /0 /-0
- Location
- Panama City Beach
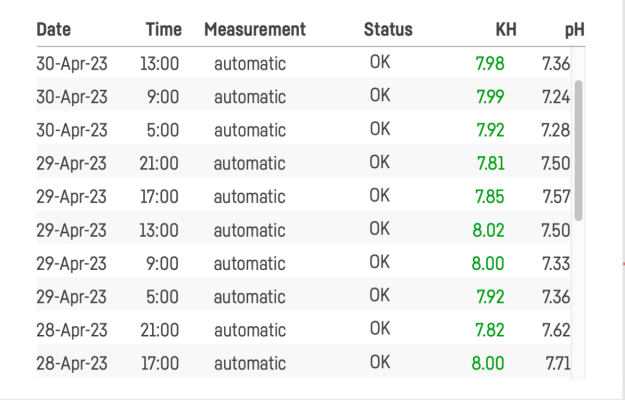
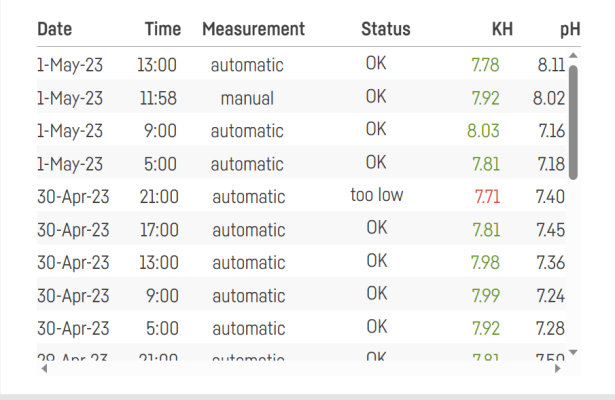
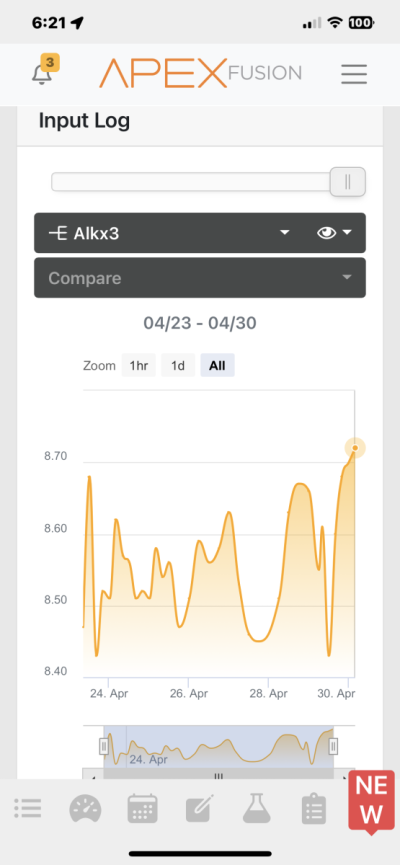
I’m dosing two part and using my Trident to test 6 times a day. Alkalinity is ranging between 8.45-8.72. I’m adjusting to try to make that more stable, but is it really necessary? Searched the forum for an answer to this question and didn’t find it. Maybe I missed it though.
thank you for your reply.
Tim
thank you for your reply.
Tim