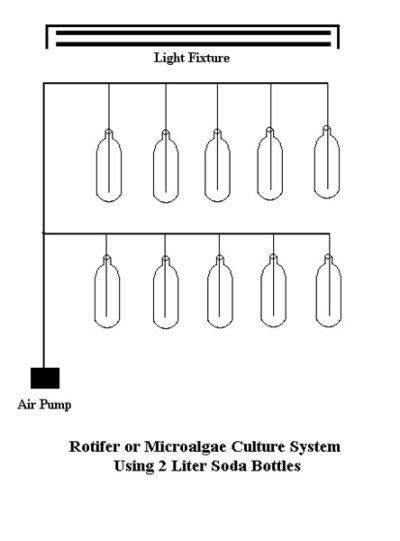
Rotifer Culture
C. Jay HemdalBrackish water rotifers, (Brachionus plicatilis) are a useful food source for certain invertebrate aquarium animals, have been used as bio-assay animals, and are a virtual necessity when raising larval clownfish (Amphiprion sp.). Many culture methods have been presented, some work well but are either costly to implement, or extremely labor intensive. Other less cumbersome methods may prove unreliable, with culture contamination being a common reason for failure.
After testing various techniques for culturing Brachionus sp. rotifers, this technique was developed to the point where catastrophic culture crashes are virtually non-existent, yet rotifer production is still high enough to raise larval clownfish and supply to filter feeding invertebrates.
Materials:
Micro algae disks (Chlorella, Nannochloropsis, etc.)
Resting rotifer cysts (small size for raising clownfish or dottybacks)
Plankton collector (plankton sieve)
Artificial rotifer food
Modified Guillard's F2 algae nutrient formula
2-liter clear soda bottles with caps (12 - 20)
Air supply and airline tubing
Fluorescent lights
Incandescent lights
Hanging tags to label bottles
Rotifer log sheet
Preparation:
1) Make 10 to 15 hanging tags for around the neck of the soda bottles. Use nylon cord and 2" x 2" white plastic and write a unique code on each one (i.e. the letters of the alphabet, A through Z and then AA to ZZ if more are needed).
2) Select the culture site; install lighting so that it is about 18" above the top of the soda bottles. Two 48" Fluorescent lights and two 100-watt incandescent spotlights are sufficient for a culture area of about 2' by 4'. A photoperiod of 16 hours per day is best.
3) Install an air supply so that there are at least 20 separately controlled airlines, which end in 15" of 3/16" rigid aquarium tubing.
4) Prepare 10 - 15 soda bottles: remove labels, drill 1/4" hole in the caps and install the airlines through the cap holes. An alternative method is to forgo using caps, and plug the necks of the bottles with polyester batting. Either way, the intent is to keep protozoan contaminants from entering the cultures. The soda bottles should either be new, or if previously used for rotifer culture, have been sterilized with a 1:10 bleach and water solution and rinsed very well.
5) Fill 4 bottles with synthetic seawater at a specific gravity of 1.014 (use tap water, not aquarium water to mix the water used to start the bottles with, as it is effectively sterile).
6) Add algae nutrient to the 4 bottles as called for in the instructions. Then, add some water to the surface of one micro algae disk, rub your finger across the agar to suspend the algae cells. Add 1/2 of the resulting green water and 1/4 to each of two other bottles. Log each bottle number with the algae start date.
7) Wait two days and repeat step 6. Within another 3 to 6 days, you will see the original bottles start to turn darker green as the algae culture starts to grow. At this point, if you are interested in only culturing micro algae, just continue in this fashion. If you wish to grow rotifers using this algae as food, then add one vial of resting rotifers to the darkest bottle, and 1/2 a vial to each of the next two darkest bottles. Log each bottle as to the date and amount of resting rotifers added. Adjust the airflow to each bottle as needed - pure algae cultures do well with a high airflow, while maturing rotifer cultures seem to do better if the air supply is turned down a bit.
8) Wait at least two days, and sub-culture two new algae bottles using 100 ml from the second set of bottles from step 7 (the ones that have NOT had rotifers added yet). Watch the bottles to which resting rotifers have been added for signs of clearing. This means that the rotifers are actively reproducing, and eating the algae culture. When a bottle becomes almost clear, either harvest the rotifers by filtering them, or feed them artificial rotifer food until you need the bottle (this keeps them from starving). When a bottle is harvested, either sterilize it, or use a new bottle and set it up as in the first part of step 8, using pure algae culture (no rotifers). Airlines and caps that have been in contact with culture water that has held rotifers must be sterilized prior to re-use as components of the algae-only cultures.
9) Once you have some bottles with newly added algae sub-cultures, some with older algae cultures, and some with active rotifers, you can begin sub-culturing algae cultures with cultures that have living rotifers in them (thus avoiding the need for any additional resting rotifers).
Hints:
Always have at least two bottles at the same stage of the culture process. That way, if a culture becomes contaminated, you can rely on the other bottle to keep things moving along. Knowing what bottles were sub-cultured from what bottle is important in tracking, and isolating any sort of protozoan or bacterial contamination. Use the log sheet to watch for this - a "bad" bottle is scratched from the records.
If you wish to harvest the micro algae for use in feeding filter feeders, you need to separate the algae cells from the culture water. Various techniques have been used to do this from centrifuging the culture to filtering it. The various algae species require different methods depending on their cell size. Micron filtration may be required for small algae such as Chlorella. For Nannochloropsis and larger species, it may be possible to extract a good portion of the culture using doubled up paper coffee filters.
Keep bottles with rotifers in them physically isolated from the pure algae cultures - airborne contamination may occur.
Learning how to "juggle" the bottles takes time, if you aren't careful, the bottles get out of "sync", and you might end up with all algae bottles, or no bottles that don't already have rotifers added to them, making them useless for sub-culturing new algae bottles.
Always work with the pure algae cultures first and then the cultures inoculated with rotifers. This avoids the chance of "back contamination" of the algae cultures with viable rotifers.
It helps to have a few bottles set up with water and nutrients a day or so before inoculating them with the algae - something about letting them aerate tends to "age" them and make it a more hospitable environment for the algae cells.
As each bottle is moved through the process, it is physically moved from left to right across the culture table. In some cases, a bottle may not "mature" at the proper rate, and it is "held back" until it shows the proper response for it to go on to the next stage. Any bottle that shows unusual response, i.e., early clearing after live rotifers are added, failure to produce a dense algae culture, etc., are scrapped and taken out of the loop, and a back-up bottle is brought into play.
With a bit of experience, one can predict the need for rotifers at the facility while avoiding the odd bottle that "fails to perform". If a higher level of rotifers is predicted, one can, with about a week's time, meet this need by doubling or trebling the number of bottles established at an earlier stage.
Supplies: http://floridaaquafarms.com/shop/